Future-Proofing Your Medical Affairs Organization
OBJECTIVES: Identify the major trends and drivers towards digital transformation and drive Medical Affairs towards a digital future.
OBJECTIVES: Identify the major trends and drivers towards digital transformation and drive Medical Affairs towards a digital future.
Download the Full Article Here
By João D. Dias1, Cesar Sanz Rodriguez2, Raphael T.B. Tan3
1 Medical Affairs & Clinical Development, Haemonetics S.A., Switzerland, Medical Affairs Professional Society (MAPS) MedTech Focus Area Working Group (FAWG) EMEA Co-Lead
2 Medical Affairs, MSD International GmbH, Switzerland, MAPS MedTech FAWG EMEA Co-Lead
3 International Medical Executive Consultants (IMEC), The Netherlands, MAPS MedTech FAWG member

This article provides an overview of the new MedTech European regulatory environment and opportunities for the Medical Affairs function to evolve and bring value to the respective organizations. The European Regulations ask for an increased effort from manufacturers to generate and communicate clinical evidence on the safety and performance of their Medical devices, In vitro-diagnostics and Drug Device Combinations. In conjunction with increased quality standards they make a compelling case for Medical & Scientific Governance with a prominent role for Medical Affairs in many pre- and post-market processes. A transformation of Medical Affairs into a strategic business function makes the medical device industry an exciting place to be for Medical Affairs professionals.
For over two decades, Medical devices and In-vitro Diagnostics have been regulated in Europe by Directives for MEDICAL DEVICES (MDD) and IN VITRO DIAGNOSTICS (IVDD)1,2,3 that were published in 1993 and 1998 respectively. A separate Directive for Active Implantable Medical Devices (AIMD) was published in 1990 with a last revision in 2009. According to these Directives, devices are approved for the European Single Market only after having obtained CE Mark for which manufacturers need to demonstrate conformity to essential requirements relating to the device’s performance and safety for patients and users. After public consultation by the European Commission in 2008 it became clear that an update of the Directives was needed, one reason being the simple fact that new technologies such as companion diagnostic devices were not yet covered. The need for revision gained traction after incidents with breast implants, transvaginal meshes around 2009 and metal-on-metal hip prostheses a couple of years later. Eventually, the revision process that started in 2012 resulted in the Medical Device Regulation (MDR) in which MDD and AIMD were combined and the In Vitro Diagnostics Regulation (IVDR)4,5. The new Regulations were published in 2017, with May 25, 2017 as the official date of entering into force. A transition period to full implementation of the MDR and IVDR was allowed for three and five years respectively, which means the MDR applies from May 26, 2020 and IVDR from May 26, 2022.
So far so good… and then the COVID-19 crisis struck Europe, right at the moment when the medical device industry and notified bodies are transitioning to the new Regulations. Therefore, in order to “take the pressure off national authorities, notified bodies, manufacturers and other actors so they can focus fully on urgent priorities related to the coronavirus,” the European Commission has decided to move back the date on which the new MDR would fully apply by one year, to 26 May 2021. MedTech Europe, the European trade organization of medical device manufacturers, has advocated for a similar delay for the IVDR.
Since the early days of the global COVID-19 crisis the medical industry has made a so far unseen effort in finding (bio-)pharmaceutical solutions, developing vaccines and reliable test kits. Simultaneously, in an attempt to address the relative shortage of masks and intensive care equipment such as ventilators, traditional medical device manufacturers ramped up production. Although regulators accommodate the surge of new devices by fast tracks and exemption rules, new devices are subject to meticulous assessments of performance and safety. And rightly so, since national policies to curb transmission of the virus rely on the quality of diagnostics and personal protection equipment. Especially important is the scrutiny in assessing new medical devices which are intended to be used in the management of the most vulnerable and severely ill COVID-19 patients who end up in hospitals and ICUs.

In general the new Regulations devices, improve traceability and transparency and define stricter requirements to clinical evidence and post-market surveillance. The MDR now also regulates devices for cosmetic purposes such as colored contact lenses and cosmetic implant devices. The introduction of Unique Device Identification should improve traceability, and transparency is created by the European Databank of Medical Devices (EUDAMED) where all mandatory regulatory documentation on each device is kept and updated. Manufacturers must re-certify devices in accordance with the new regulations and update their technical documentation accordingly with special attention to higher clinical requirements for class III and implantable devices. A shift of focus from a mere pre-market perspective towards a life-cycle approach also includes stricter requirements regarding post-market surveillance, post-market clinical follow-up and vigilance.
This brings us to the Clinical Evaluation, which lies at the basis of CE mark approval and the life-cycle approach. It is defined as “a systematic and planned process to continuously generate, collect, analyze and assess the clinical data pertaining to a device in order to verify the safety performance, clinical benefits of the device when used as intended by the manufacturer.” The Clinical Evaluation process is described in a MEDDEV (MEDical DEVices) guidance document. MEDDEVs promote a common approach to be followed by manufacturers and notified bodies that are involved in conformity assessment procedures. Although these MEDDEVs are not legally binding, it is expected that their guidance be followed, ensuring the uniform application of the various elements of the directives/regulations. MEDDEV 2.7/1 rev. 46 is the guidance document with regards to Clinical Evaluation and is prescriptive on the process, the required qualification of the evaluators and the contents of a Clinical Evaluation Report (CER).
The CER should “describe the intended clinical benefit and provide evidence of safety and performance” where performance is defined as “the ability of a device to achieve its intended purpose as stated by the manufacturer.” This report describes the risk profile of the device based on the technical documentation and provides an appraisal of all available clinical data related to safety and performance. Any evidence gaps and residual risk need to be addressed in Post-Market Clinical Follow Up (PMCF) studies to demonstrate long-term performance safety. The results from the intensified surveillance are laid down in the Periodic Safety Update Report (PSUR, mandatory for Class IIa/b and III) and Summary of Safety and Clinical Performance (SSCP, for implantables and Class III). These documents must be uploaded in EUDAMED, which allows public access to the SSCP.
Although European market approval of new devices can still be obtained by referring to clinical data of predicate equivalent devices, the MDR explicitly lists criteria by which equivalence can be claimed from a technical, biological and clinical perspective. The equivalence under the MDD was less well defined. Under the MDR, the predicate device must have a similar design, use the same materials and come in contact with the same tissues and body fluids, and be used for the same clinical indications. If a device does not meet these criteria, manufacturers need to generate their own clinical evidence with appropriate clinical investigations.
CE Marking requires Notified Body involvement for most medical device classes and is related to implementation of a Quality Management System, which must include plans pertaining to Clinical Evaluation, Post-Market Surveillance (PMS) and Post Market Clinical Follow-up (PMCF). For non-sterile Class I devices, manufacturers can conduct the conformity assessment themselves and basically self-certify. For Class I devices that are sterile, measuring or reusable surgical instruments as well as for all higher device classes, oversight of a Notified Body is required. In the end, it is the Notified Body that issues a CE Marking Certificate and ISO 13485 Certification. Only with these certificates in place the manufacturer can prepare a Declaration of Conformity and put the CE Mark sign on their products and labelling.

In Vitro diagnostics (IVDs) are medical devices with which tests are performed using human specimens such as urine or blood. Familiar examples of such devices are pregnancy tests and tests for determining the level of glucose or cholesterol in the blood. The current directives distinguish between medium risk and high-risk lists of IVDs. Those IVDs not captured in these lists are automatically classified as low risk. Obviously more stringent market authorization procedures with Notified Body oversight apply to high risk IVDs. Whereas in the past only a minority of IVDs required involvement of a Notified Body, under the new classification an estimated 90% will now require it. The IVDD had some gaps and this binary system was thought to be no longer sufficient. Hence, like for the MDR, a risk-based approach classification was introduced based on the severity of the disorder tested for and possible consequences of an incorrect test result. Instead of two lists, the new IVDR now distinguishes four categories, Class A (lowest risk), Class B, Class C, and Class D (highest risk) and dictates that Class B and above IVDs will require oversight from a Notified Body as part of their conformity assessment.
Aside from the risk-based classification, it will not surprise you that the new IVDR has more features in common with the MDR. As is the case for medical devices, the IVDR requires clinical evidence and post-market performance follow-up. This will require a Performance Evaluation plan and report for all IVD Classes, which will describe how to demonstrate scientific validity, analytic performance, and clinical performance.

Some medicines are used in combination with a medical device, usually to enable the delivery of the medicine. In the European Medicines Agency (EMA) view, if the principle intended action of the combination product is achieved by the medicine, the entire product is regulated as a medicinal product under Directive 2001/83/EC7 or Regulation (EC) No 726/20048. However, MDR’s Article 117 brought some relevant additions. First, two categories were defined: (a) Integral, where the medicinal product and the device form a single integrated product (e.g. pre-filled syringes and pens) and (b) Co-packaged, where the medicinal product and the device are separate items contained in the same pack (e.g. reusable pen for insulin cartridges). Next, Article 117 also incorporated some relevant amendments to Directive 2001/83/EC to ensure combination products comply with the medical device legislation. Per MDR’s Article 117, the marketing authorization application should include a CE certificate for the device or an opinion from a Notified Body on the conformity of the device (except for non-sterile, non-measuring and non-reusable surgical Class I devices).
Probably as a reaction to the rapid growth of combination products in recent years and the need to bring further clarity in this area, in June 2019 EMA released for public consultation a draft Guideline on Quality Requirements for Regulatory Submissions for Drug-Device Combinations9. The aim of this Guideline is to clarify expectations laid down in Directive 2001/83/EC and address the new obligations in the MDR. EMA makes it clear that the Notified Body assessment and marketing authorization review would not result in duplicate assessments. The former will review the device alone, while the latter will ensure the safety and efficacy of the drug are not compromised by the inclusion of the device part. The consultation period ended in August 2019 and EMA is now due to finalize the Guideline in the second quarter of 2020.
It is also worthwhile making a reference to medical devices which may contain an ancillary medicinal substance to support the proper functioning of the device (e.g. drug-eluting stents). These products should comply with the medical device legislation. Yet, the manufacturer should also seek a scientific opinion from EMA on the quality and safety of the ancillary substance if it is derived from human blood or human plasma, or if it is within the scope of the centralized procedure for the authorization of medicines. For other substances, the Notified Body can seek the opinion from EMA or a national competent authority. Of note, EMA has recently issued a Consultation Procedure for Ancillary Medicinal Substances in Medical Devices.
Companion diagnostics are seen by EMA as in vitro diagnostic tests that support the safe and effective use of a specific medicinal product, by identifying patients that are suitable or unsuitable for treatment. Applicable regulations were discussed above. Yet, before the Notified Body can issue CE Marking, it must seek a scientific opinion on the suitability of the companion diagnostic to the medicinal product concerned from EMA or a national competent authority, as appropriate. Similarly, a scientific opinion would also be needed for some other medical devices made of substances that are absorbed by the human body to achieve their intended purpose. These devices are normally introduced into the human body via an orifice or applied to the skin. Last, we should not forget the so called “borderline products”. These are complex healthcare products for which there is uncertainty over which regulatory framework applies. Common borderlines are between medicinal products, medical devices, cosmetics, biocidal products, herbal medicines and food supplements. The European Commission publishes the ‘Manual on borderline and classification in the Community regulatory framework for medical devices’ which provides examples and recommendations for determination of classifications. National competent authorities classify borderline products either as medicinal products or, for example, as medical devices on a case-by-case basis based on the product’s composition and constituents, its mode of action and its intended purpose. This determines the applicable regulatory framework.

The new European regulatory environment (MDR, IVDR and EMA guidance on Drug Device Combinations) in conjunction with expanded quality standards for MedTech products and a rapidly changing reimbursement landscape are intensifying the need for a Medical Affairs role in the product lifecycle management. Furthermore, the increased trade organization’s guidelines as well as a more complex competitive environment in which the direct comparator might not be another MedTech product but instead a Drug or a Drug Device Combinations, all work favorably for Medical Affairs to step up its game and demonstrate its value in many regards.
The traditional competencies of Medical Affairs are still required to produce the new mandatory regulatory deliverables (Clinical/Performance Evaluation Report, Periodic Safety Update Report, Summary of Safety and Clinical Performance). However, more than ever Medical Affairs involvement needs to be formalized for various other key processes ranging from product development to device application. In order to give real meaning to patient and customer centricity, the inclusion of Medical Affairs contribution is indispensable with regard to, for instance, risk analyses, claims development and identifying user training needs.
In addition, medical and clinical research functions must lead in the development of coherent and affordable clinical research programs that serve regulatory compliance, reimbursement and commercial adoption purposes. The increased effort the companies must make to generate clinical evidence will undoubtedly put strain on human and financial resources. The careful planning and design of studies should avoid waste in time and money while providing the evidence the business needs in a timely fashion. Therefore, as non-inferiority assessments are giving space to superiority assessments, an increased is expected in reliance for regulatory purposes on less conventional evidence generation strategies such as registries, collaborative research and investigator lead studies.
Furthermore, ensuring the safe application of current devices, medical communication and interactions with healthcare stakeholders and health authorities, collecting and weighing medical intelligence on future directions in healthcare all require medical scientific oversight. The medical scientific oversight will be fundamental in the advancement of the company’s innovation agenda as the bar is higher than ever, as are the associated entry and maintenance costs.

The new environment makes a compelling case for installing a structure for Medical and Scientific Governance and a proactive strategic role for Medical Affairs. This in turn opens the discussion on how to develop and organize competencies around organizational capabilities that relate to the development of innovative and relevant devices, the substantiation of medical-clinical and health economic claims and ultimately oversight to ensure safe and appropriate use. Thus, we argue that now is the time to engage in captivating thought exercises about whether a company’s fabric with a prominent role of Medical Affairs adds to the organizational capability and resilience to handle current and future challenges.
This publication represents the consensus opinions of the authors and various members of MAPS, but does not represent formal endorsement of conclusions by their organizations
1. EU Council Directive 93/42/EEC of 14 June 1993 concerning medical devices. EUR-Lex; an official website of the European Union. Published July 12, 1993. Accessed August 27 2020.
2. EU Council Directive 98/79/EC concerning in-vitro diagnostic medical devices. EUR-Lex. Published December 7, 1998. Accessed August 27, 2020.
3. EU Council Directive 90/385/EEC of 20 June 1990 on the approximation of the laws of the Member States relating to active implantable medical devices, EUR-Lex, Published July 20, 1990. Accessed August 27, 2020
4. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices. EUR-Lex. Published May 5, 2017. Accessed Aug 27, 2020.
5. Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices. EUR-Lex. Published May 5, 2017. Accessed Aug 27, 2020.
6. European Commission. MEDDEV 2.7/1 rev. 4 – Clinical Evaluation: A guide for manufacturers and notified bodies. Website of the European Commission. Released June 2016. Accessed August 27, 2020.
7. EU Council Directive 2001/83/EC of 6 November 2001 on the Community code relating to medicinal products for human use. EUR-Lex. Published Nov 28, 2001. Accessed Aug 27, 2020.
8. EU Regulation (EC) No 726/2004 of 31 March 2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency. EUR-Lex. Published April 30, 2004. Accessed Aug 27, 2020.
9. European Medicines Agency – draft guideline on the quality requirements for drug-device combinations. EMA Website. Published June 3, 2019. Accessed Aug 27, 2020.
This MAPS webinar features Todd Neuville, Worldwide Leader – Life Sciences, Amazon Web Services presenting a case study of Amazon’s approach of “working backwards,” in discussion with our expert panel.
Medical Affairs’ role in securing a bright future for pharma is undeniable, yet many before have struggled to bring forth concise and consistent descriptions that communicate the full range of benefits and expertise that Medical Affairs (MA) brings to the table. As a result, the wider understanding of MA’s role is not where it should be. To that end, this white paper explores the role and value of MA, backed up with commentary from industry leaders, as we define clear pillars of MA that communicate its true value. What’s more, we propose a short elevator pitch that MA professionals can use to quickly yet succinctly describe the importance of MA for every successful pharma venture in the future.
Through this course you will recognize terms such as artificial intelligence (AI), machine learning (ML), deep learning, and neural networks to arrive at best decisions and insights needed for success.
OVERVIEW:
The COVID-19 pandemic is affecting Medical Affairs professionals around the world, and each team is responding in different ways. During this live Global Town Hall, we will discuss how Medical Affairs can continue to function during the COVID-19 pandemic, as well as share industry best practices and consider how Medical Affairs could change post-COVID-19. Through patient-focus and peer-to-peer relationships with HCPs, Medical Affairs professionals have a unique role in ensuring the safe and effective use of medicines, vaccines and medical devices, and are also positioned to bring invaluable insights from the field into ongoing R&D. By strengthening our understanding within the pharmaceutical industry of how Medical Affairs can support HCPs and patients in these times, this Town Hall will explore how the role of Medical Affairs can be expanded to maintain quality in a rapidly evolving clinical environment, helping to achieve industry-wide alignment on the issue and potentially saving lives.
Join this webinar for insights from industry-leading experts. This series including follow-on webinars will equip MAPS members and MA professionals worldwide with the tools to fulfill the opportunity for Medical Affairs strategic leadership during the Covid-19 pandemic and beyond.
MAPS Members have access to view and download the slides from this presentation.
CLICK HERE TO VIEW AND DOWNLOAD SLIDES IN THE COMMUNITY PORTAL
SPEAKERS:
Rachele Berria
Vice President and Medical Head, US BioPharmaceuticals
AstraZeneca
Eric Mortensen
Head Gastrointestinal Clinical Development, R&D
Janssen Immunology
Tamas Koncz
Chief Medical Officer, Inflammation and Immunology
Pfizer
Isma Benattia
VP, Europe Medical Affairs
Amgen
Terry Griesing
VP, Head of North America Medical Affairs, Internal Medicine
Pfizer
Ann Hartry
VP, HEOR
Lundbeck
Audrey Krolicki
Senior Director, Head of Scientific Publications
Astellas
Danie du Plessis
Executive VP Medical Affairs
Kyowa Kirin International
AGENDA
| Time | Title | speaker |
| 9:00–9:10 EDT | The Opportunity for Medical Affairs Strategic Leadership | Chair: Tamas Koncz |
| 9:10–9:20 EDT | Evidence Generation: Ensuring Speed and Quality During Rapid Decision-Making |
Ann Hartry |
| 9:20–9:30 EDT | Evidence Dissemination: The New World of Publications and Virtual Congresses | Audrey Krolicki |
| 9:30–10:05 EDT | Audience Q&A and Panel Discussion
What has changed for Field Medical? What hasn’t changed? What are the needs now, and in the future? How can the return-to-field be balanced with continued virtual interactions? What are the key adaptive strategies, innovations and practices? |
Full Expert Panel |
| 10:05–10:15 EDT | Closing: The Opportunity for MA is Here and Now | Tamas Koncz |
As a result of the global COVID-19 outbreak, field teams are no longer able to visit HCPs face-to-face. During this Webinar, we will explore the skills and challenges around effective remote engagement and share hints and tips to help you access, engage and follow-up with HCPs remotely.
Topics Discussed in the Town Hall Include:
-Managing Individual Performance Remotely
-Virtual Leadership Mindset & Skills
-Effective Team Engagement in the Virtual Space
-Engagement of KOLs Virtually
SPEAKERS:
Cezary Statuch
VP, Medical, Intercontinental Region
Biogen
Greta James-Chatgilaou
Field Medical Strategy and Execution Director
Biogen
Alan McDougall
VP, Head of Medical Affairs, International Markets and Greater China
Astellas
Qasim Ahmad
Corporate Officer/VP, Head of Japan Medical Affairs, OBU
Novartis
WATCH IT HERE:
If you have difficulty viewing or are unable to view full screen above, please click here.
By: Simon Kyaga1; Keith Morris2; Kiely Flanigan3
1Global Medical Lead, Psychiatry, Servier; 2Executive Managing Director, Scientific and Medical Affairs, Syneos Health; 3Director, Medical Affairs Syneos Health
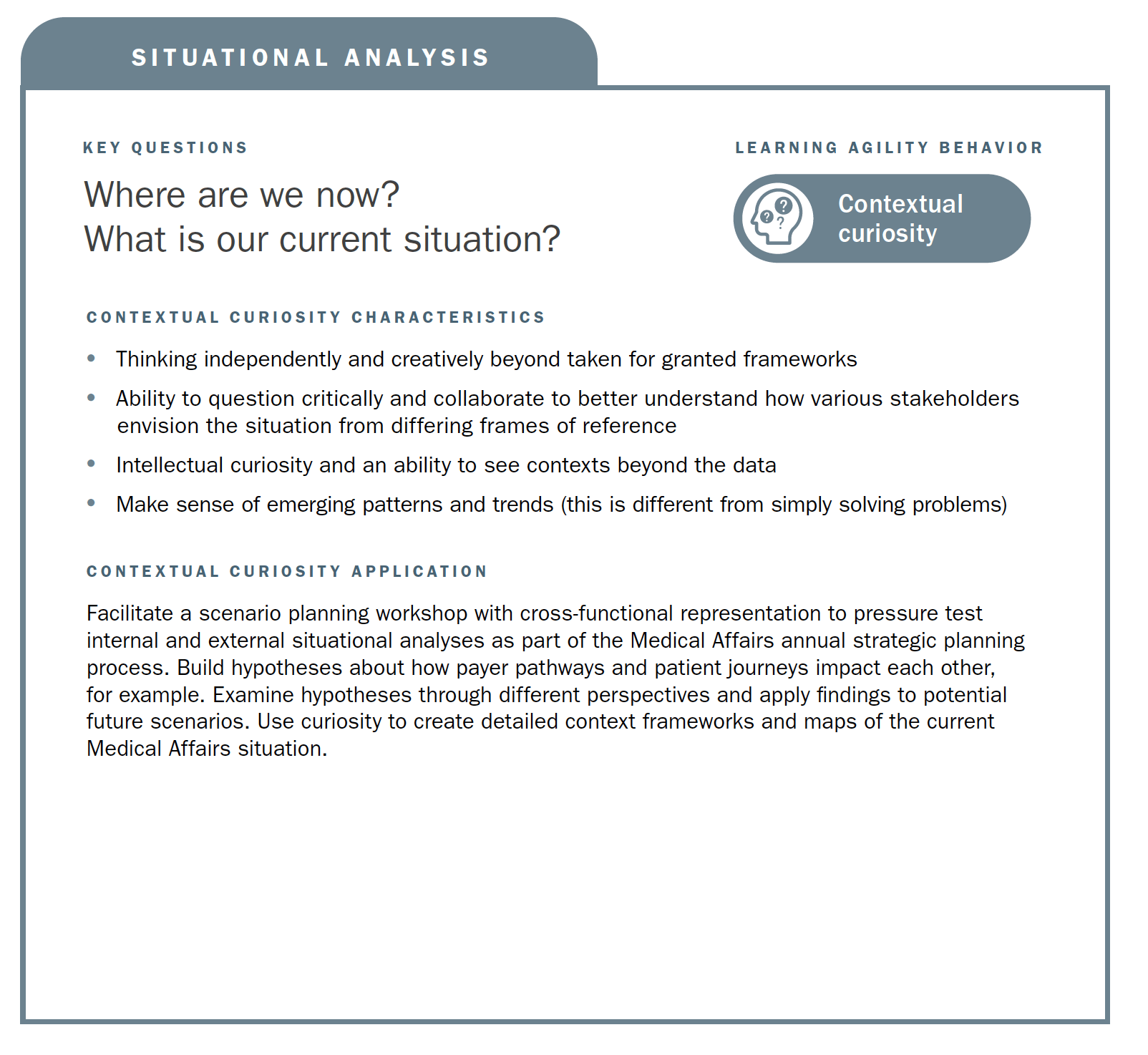
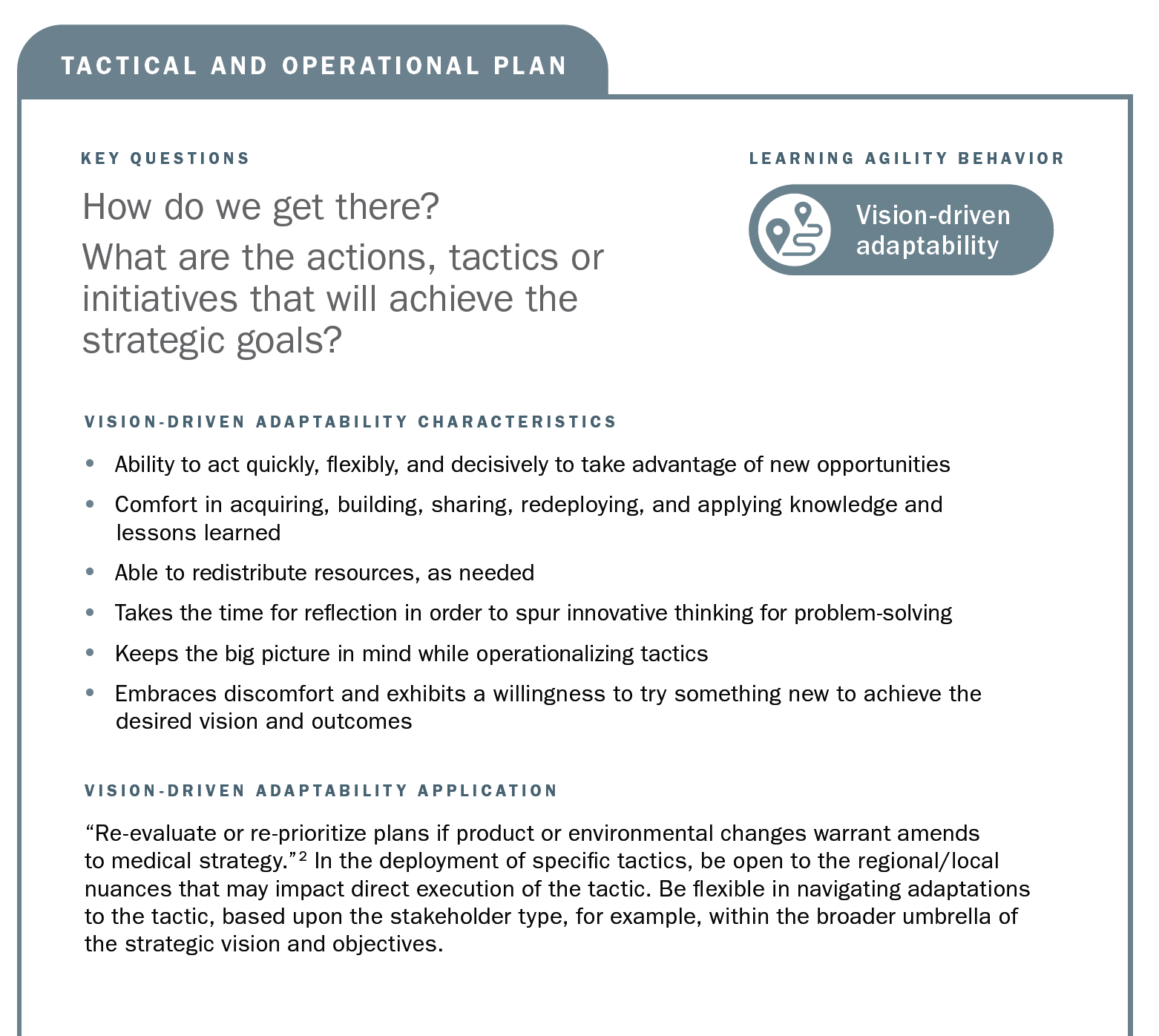
This article aims to position learning agility as an emergent capability that supports the future-proofing of Medical Affairs strategic planning processes and outputs. In essence, learning agility is a set of skills, competencies, and mindsets that support our capability of “knowing what to do when we don’t know what to do.”1 Our position is that learning agility is a capability that should be developed internally and applied to the development and operationalization of strategic plans. Through the enablement of learning agile behaviors, the approach to strategic plans can be made with an eye toward ongoing reflection and updates. We define four descriptive behaviors (contextual curiosity, vision-driven adaptability, educated risk taking, and accountable learning) that match up to MAPS best practices in strategic plans and then discuss how to apply those learning agility behaviors. We conclude with future recommendations for the development and application of learning agility.
Learning agility, strategic plan, strategic thinking, medical strategy, capabilities enhancement
The COVID-19 pandemic has accelerated the need to develop Medical Affairs capabilities in learning agility. Not unlike various industry shifts over the years that have impacted the role of Medical Affairs, we’ve experienced a shift in the way Medical Affairs organizations are responding to changes affecting the execution of strategic plans. Field Medical is learning to engage stakeholders virtually and support HCPs in new ways as they engage with their patients through new technologies. Clinical trials adopted new protocols to protect patients and sustain recruitment. Conferences and congresses were postponed. Organizations are seeking flexible resourcing models to manage downturns in business and leveraging downtime to upskill team members. All of these changes have required an openness to change and the development of new skills to learn new ways of achieving our work objectives. We are now not only shifting how we do our Medical Affairs work, but also planning for a “new normal” as we navigate doing business virtually.
Yet, however uniquely disruptive COVID-19 has been, it is still only one more example of the bucket of business disruptions that have affected the skills, knowledge, and capability needs within Medical Affairs work. At the heart of the changes asked of us and our teams is learning agility. As a core capability associated with managing ambiguity and “knowing what to do when you don’t know what to do,” learning agility is particularly relevant and useful in developing adaptive and dynamic Medical Affairs strategic plans that stand the test of change and disruption.1
Learning agility in strategic planning is important because by incorporating learning agility behaviors and mindsets into the development and implementation of Medical strategic plans, teams are better able to pivot and innovate, as needed, to changing internal and external dynamics, while remaining in alignment to the overall medical vision and business objectives.
This article aims to position learning agility as an emergent capability that supports the future-proofing of Medical Affairs strategic planning processes and outputs. Our position is that learning agility is a capability that should be developed internally and applied to the development and operationalization of strategic plans. We then define four descriptive behaviors that map to aspects of Medical Affairs strategic plans and discuss how to apply those learning agility characteristics.
Medical Affairs strategic plans include both intellectual components, such as situational analyses and medical strategies, as well as tactical components, including tactical and operational plans, and assessment and measurement metrics.2 Medical plans are important because they guide decision making across the organization and support the communication and assessment of Medical Affairs’ efforts and impact.
Medical Strategic Planning is an integral part of setting strategic direction and articulating the tactics for driving Medical Affairs value and impact for patient and organizational outcomes. But, how do you create a realistic and viable strategic plan given a VUCA (volatile, uncertain, complex, and ambiguous) Medical Affairs ecosystem? What brings Medical strategic plans to life beyond and ensures it gets referenced more than once a year in the annual planning process? How do we make the strategic plan content memorable and keep it top of mind with our key audiences? We suggest that the secret to effective Medical Affairs strategic plans is learning agility.
In a Medical Affairs context, learning agility brings key behaviors and mindsets into the Medical planning, development, and execution processes that ensure the content contains relevancy and resonance for the organization. Although variations exist between companies in terms of influences affecting the strategic planning process (e.g., preferred timing of Medical support of launches, products, therapeutic area considerations, and operational competencies versus strategic positioning priorities), the Medical Affairs strategic planning process reflects multiple stakeholder insights, business objectives alignment, and tangible data for strategic decision making. The strategic plan is not intended as a fixed manual that is reviewed once a year.
There are several descriptive behaviors team members can use in approaching the development, communication, and operationalization of strategic plans to support success:
Each of these behaviors is based on the capabilities needed to design, communicate, and execute on a strategic plan. The descriptiveness of the terms reflects a desire to position these learning agility behaviors as both foundational and aspirational. Learning agile behaviors are both critical for the here and now in performing work, but also for guiding toward the future and inspiring learning and development.
Learning agility is relatively new to the Medical Affairs scene, but it is starting to see more traction as our industry seeks to build capabilities in individuals and teams to navigate and harness the rapidly changing nature of Medical Affairs. Originally used to develop the managerial capabilities of high-potential, high- performing talent, learning agility can be applied not only at the individual level, but also at the team and organizational levels, and is associated with higher levels of organizational performance.3,4 For purposes of this article, we’ll focus on developing learning agility at the individual level and use the following definition:
Learning agility requires both adaptive readiness to change and proactive innovation in times of ambiguity.5 In essence, learning agility activates the value and impact of Medical Affairs strategic planning components (i.e., situational analysis, medical strategy, tactical and operational plans, and assessment and measurement metrics) despite change and shifting expectations internally and externally.
When it comes to Medical Affairs strategic plans, it is no longer sufficient to rely on the intellectual and tactical domains of competence. Successful Medical Affairs strategic plans reflect a collective organizational capability—an integrated representation of knowledge, skill, and mindset—that brings to life within the plan the flexibility to adapt, learn, and pivot toward changing needs. Learning agility is the “how” behind the “what” of Medical Affairs strategic plans.
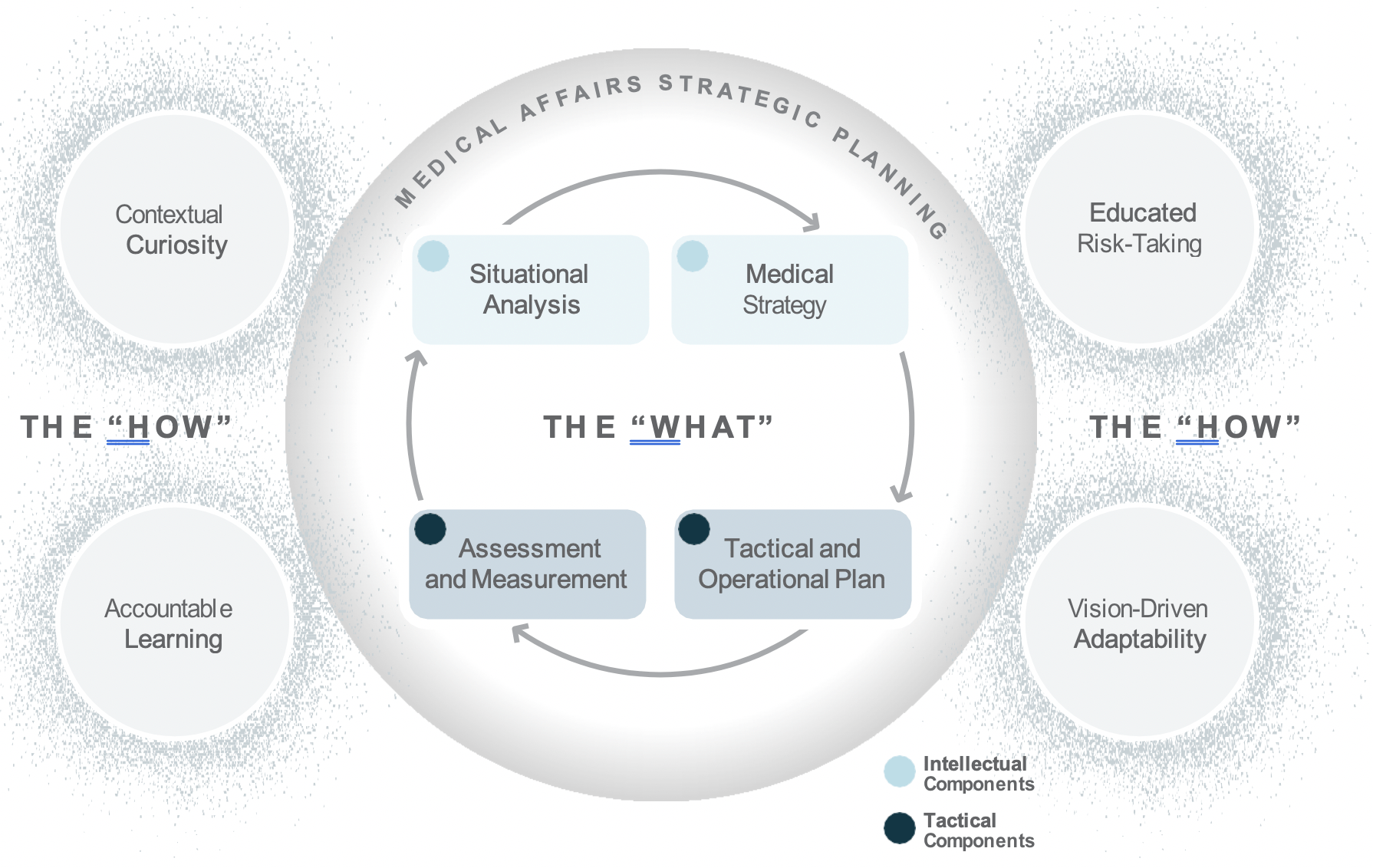
The integration of learning agility and Medical strategic planning is important to how both strategic and day- to-day operational decisions are made. This is even more important today as Medical Affairs is being asked to communicate and demonstrate its impact and value within competing priorities from more diverse and increasingly challenging internal and external stakeholder needs. In addition, due to COVID-19 disruptions, including closed conferences, Medical Affairs is forced to reconsider how to communicate and how to balance between strategy and tactics in an uncertain environment.
Using the strategic planning framework developed by MAPS, the section below looks at the fundamentals of Medical strategic planning and suggests related learning agility behaviors and mindsets that are instrumental to both intellectual and tactical outcomes. The learning agility behaviors form a kind of permeable flexibility and protection that ensures the strategic plan is created and maintained with maximum adaptiveness (as seen in the above diagram). Learning agility brings strategic plans to life and articulates specific behaviors that support the strategic plan having bigger impact through greater relevancy. A strategic plan must be relevant to have impact and the learning agility behaviors associated with MAPS’ four elements of strategic plans makes them applicable for the teams using them.

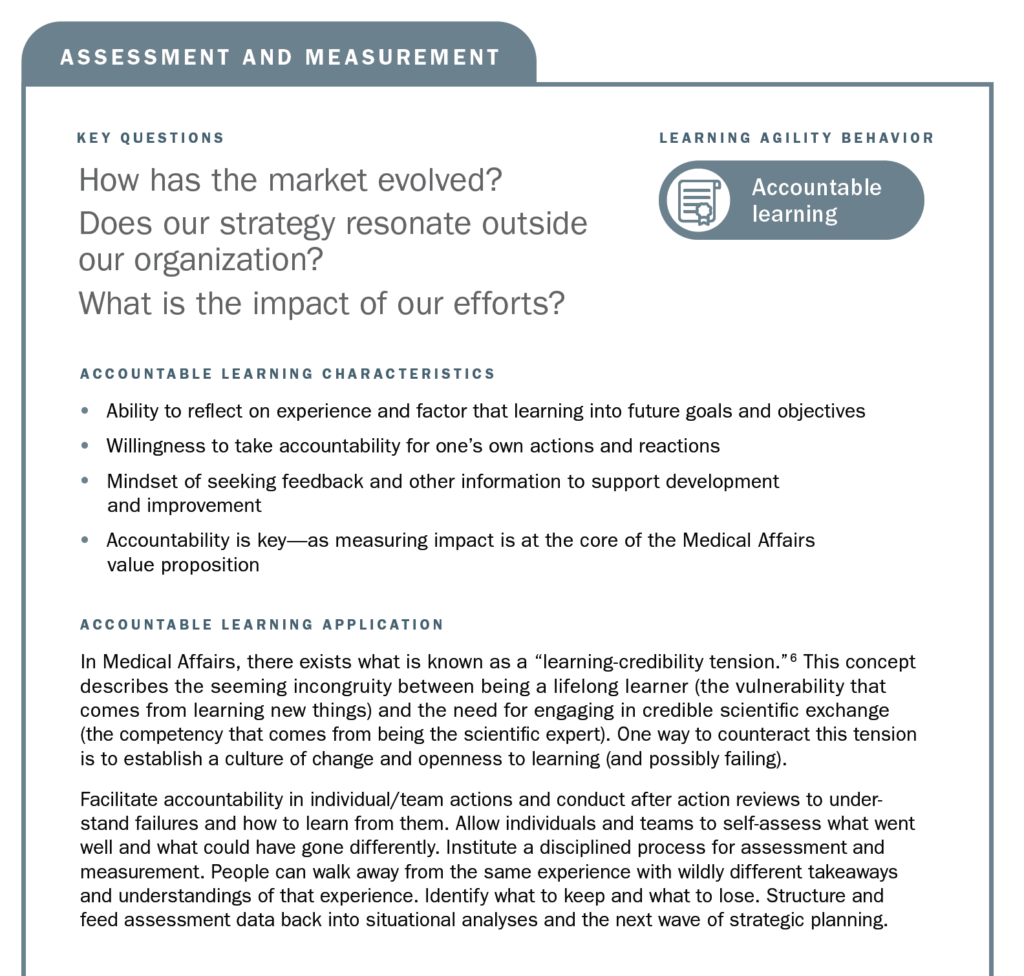
In this article, we have identified learning agility as an emergent capability that brings to life and ensures the relevancy of Medical Affairs strategic plans. Specific applications were discussed for each learning agility behavior in context to its associated strategic planning element. The importance of developing the skills and mindsets to navigate change, uncertainty, and disruption are evident, now more than ever with COVID-19, and important to developing the capabilities to harness the future, whatever may come within Medical Affairs.
1. Hallenbeck, G., & Santana, L. (2019). Great leaders are great learners: How to develop learning-agile high potentials. Center for Creative Leadership white paper, 1-16.
2. MAPS Annual Conference (2020). The importance of Medical strategic planning. Conference presentation: Miami, 1-23. Access in the Community Portal.
3. De Meuse, K.P. (2017) Learning agility: Its evolution as a psychological construct and its empirical relationship to leader success. Consulting Psychology Journal: Practice and Research, 69(4), 267–295.
4. McCann, J., Selsky, J., & Lee, J. (2009). Building agility, resilience and performance in turbulent environments. People & Strategy, 32(3).
5. Doeze Jager-van Vliet, SB, Born, MPh, & van der Molen, HT (2019). Using a portfolio-based process to develop agility among employees. Human Resource Development Quarterly, (30), 39–60.
6. Bourgoin, A. & Harvey, J-F. (2018). Professional image under threat: Dealing with learning–credibility tension. Human Relations, 71(12), 1611–1639.
Join this Webinar to better understand contemporary practices for data generation strategies; describe how to design data generation strategies, implementation of the strategies, and how to communicate new evidence; and describe how real-world evidence may be utilized to support product value including regulatory label change.
602 Park Point Drive, Suite 225, Golden, CO 80401 – +1 303.495.2073
© 2025 Medical Affairs Professional Society (MAPS). All Rights Reserved Worldwide.
