Download the Full Article
By: Lilly Stairs1; Annick de Bruin, MBA2; Diane Maloney, JD3a; Peyton Howell, MHA4; Roslyn F. Schneider, MD, MSc5; Leonard A. Valentino, MD6
1Founder & Principal, Patient Authentic; 2Director, Research Services, Center for Information & Study on Clinical Research Participation (CISCRP); 3Associate Director of Policy, US Food & Drug Administration (FDA), Center for Biologics Evaluation and Research (CBER), MAPS Patient Centricity Focus Area Working Group member; 4Chief Commercial & Strategy Officer, Parexel International; 5Principal, RozMD Patient Affairs Consulting, MAPS Patient Centricity Focus Area Working Group member; 6President and CEO, National Hemophilia Foundation (NHF), Professor, Rush University, MAPS Patient Centricity Focus Area Working Group member
aThis article reflects the views of the author and should not be construed to represent FDA’s views or policies.
Introduction
Patient centricity was a key theme that emerged in several of the plenary presentations and workshops at the March 2020 Medical Affairs Professional Society (MAPS) Annual Meeting in Miami, Florida, and is reflective of broader strategic goals in the biopharmaceutical industry. As MAPS Board Member Danie du Plessis noted in his presentation on the Value of Medical Affairs, Medical Affairs professionals are well positioned to ensure that the voice of the patient is heard in their organizations, and true patient partnership is part of the overarching strategic vision.
Two events at the Annual Meeting specifically focused on this theme and provided not only a cross-functional perspective on the current state of patient centricity in our industry but also practical examples for Medical Affairs professionals to implement patient-focused approaches in their everyday work. The sessions sparked considerable interest, and attendance was particularly high at the plenary session, with over 450 MAPS participants attending live and virtually.
- Plenary Session: Beyond Patient Centricity: True Patient Partnership in the Pharmaceutical Industry
- Workshop: Patient Engagement in the Pharmaceutical Industry: Different Perspectives
View these resources in the Community Portal.
The events were spearheaded by MAPS Patient Centricity Focus Area Working Group Co-Leads, Tricia Gooljarsingh, PhD (Momenta Pharmaceuticals) and Jamie Kistler, PhD (Parexel International), with support from Isabelle Bocher-Pianka (Ipsen). The Working Group, comprising Medical Affairs professionals, as well as representatives from nonprofit patient advocacy organizations, regulatory agencies (US Food and Drug Administration [FDA]), and patient-focused consultancies, was established 2 years ago to provide a platform to advance the patient centricity agenda in the MAPS organization and share best practices for pharmaceutical engagement with patients.
This article provides highlights from the plenary session, which included a multidisciplinary panel of speakers with broad and deep expertise on the topic. The cross-functional approach reflected the collaboration that is foundational for true patient engagement and partnership in the pharmaceutical industry. The faculty members represented a range of organizations and interests, but all share a passion for improving patient engagement and empowering Medical Affairs professionals with the information and tools necessary to “move the needle” in our industry. A more comprehensive manuscript is in development with the goal of expanding on the information provided in the plenary session and extending the reach of these valuable insights and perspectives to a broader audience (including patients, Medical Affairs professionals, and healthcare providers). This article will feature interviews with the speakers to provide further depth and insights, including case studies and examples of current best practices.

The plenary session was moderated by Dr Len Valentino, President and CEO of the NHF, who introduced the session with the mantra “not for patients without patients.” Len provided an overview of the agenda and learning objectives
- Review increasing public and patient awareness and education around clinical trials
- Discuss the impact of patient engagement from a patient/patient advocate perspective
- Outline current and future FDA Center for Biologics Evaluations and Research (CBER) initiatives to enhance patient and public engagement in the drug development process
- Identify best practices for pharmaceutical/medical device industry engagement with patients (appropriate initiatives that align with patient needs)
As both a clinician and a leading patient advocate, Len brought his own insights and experience to the discussion as to why patient centricity and collaboration among multiple stakeholders is so critical.
“All of us are here because our products or devices are used by patients. We need to understand what is most important to patients in our drug and device development, and collaboration is key to get this information. Not just collaborating internally with commercial organizations, HEOR, Clinical Operations, and Clinical Development but with the most important stakeholder—the patient. The patient voice should be part of all those programs and weaved through each stage of the drug development plan.”
He added that Medical Affairs is uniquely qualified to influence or directly drive patient centricity as “many of us are clinicians, and therefore our background has always been focused on the patient and patient care.”

In the opening presentation, Annick provided insights from a biennial global research study conducted by CISCRP that included 12,450 respondents, of whom 3600 were actual clinical study volunteers. The 2019 CISCRP Perceptions and Insights Study monitored trends in perceptions in clinical research among the general public and collected information about clinical trial experiences.1
Interestingly, perceptions around clinical trials have not changed substantially since the initial study in 2013, and, while people consider research to be very important, this often does not translate to active participation in clinical trials. This remains a key issue for the research community.
Annick shared insights from the data regarding what channels are most effective in overcoming barriers. A patient’s relationship with their doctor was found to have the greatest impact on willingness to participate. Technology and decentralization of studies also provide opportunities to reduce patient burden and involve more patients in clinical trials. For example, travel time to clinics was reported as a major burden impacting participation, especially among young people (Figure 1).
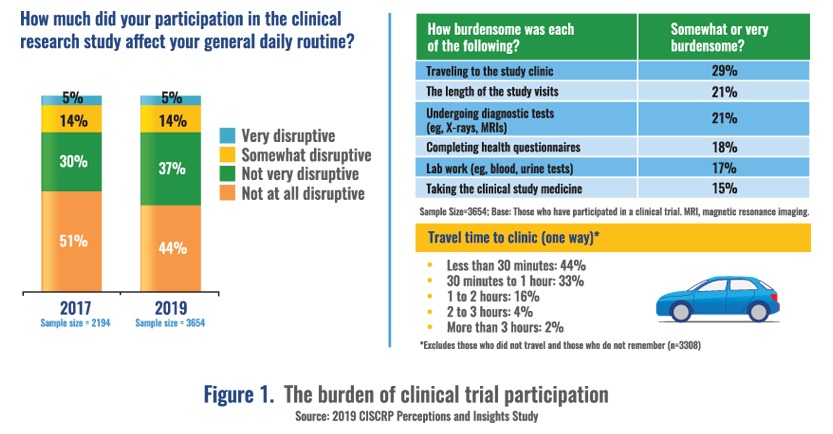
Annick concluded with the advice, “No one size fits all. Everybody wants different options to make sure clinical trial participation fits into their lives with minimal disruption.”

Annick’s perspective on the current state of patient and public involvement and awareness of clinical trials was followed by a very personal insight into the key success factors in engaging with patients. Lilly Stairs was diagnosed with 3 autoimmune conditions—Crohn’s disease, psoriatic arthritis, and psoriasis—all within a 6-month period, and her experience inspired her to become a patient advocate and engage with other patients, patient advocacy organizations, and pharmaceutical industry partners to affect change.

In her presentation, Lilly emphasized the need for the pharmaceutical industry to learn from other consumer-facing industries. In particular, she highlighted that we need to ensure a clear understanding of the end user’s needs, something she feels we have not traditionally done consistently in healthcare. She also stressed the importance of engaging with all patients and not just advocates.
“While it’s important to talk to the advocates who are able to give you high-level strategic input, it is also really critical to engage with the everyday patient who will have valuable insights and feedback. Patients are ready to engage around their healthcare. They are excited and eager to share their thoughts and experiences to move the needle and create a better tomorrow for all patients.”
Her presentation included the key principles of how we should engage with patients (Figure 2).
The importance of metrics was emphasized throughout the presentation, as well as demonstrating the value of patient advocacy to the business. Lilly provided examples in which patient advocacy work has resulted in a clear measurable impact.
“During my tenure as Head of Patient Advocacy at Clara Health, I was asked to look at a pre-screener for a lupus clinical study that was struggling to complete enrollment. It was so confusing and complex that patients were not getting through the screening stage. By working with autoimmune patients to improve the screener alongside other patient-centric tactics, recruitment speed was increased by 400%, saving a trial on the brink of shutting down.”

This presentation provided a unique opportunity to gain an insight into the work that the FDA is doing around patient centricity. Diane emphasized that patient engagement is important across all organizations in the FDA, from the Commissioner’s Office to the Product Centers, and they collaborate and meet regularly on cross-cutting patient issues (Figure 3).
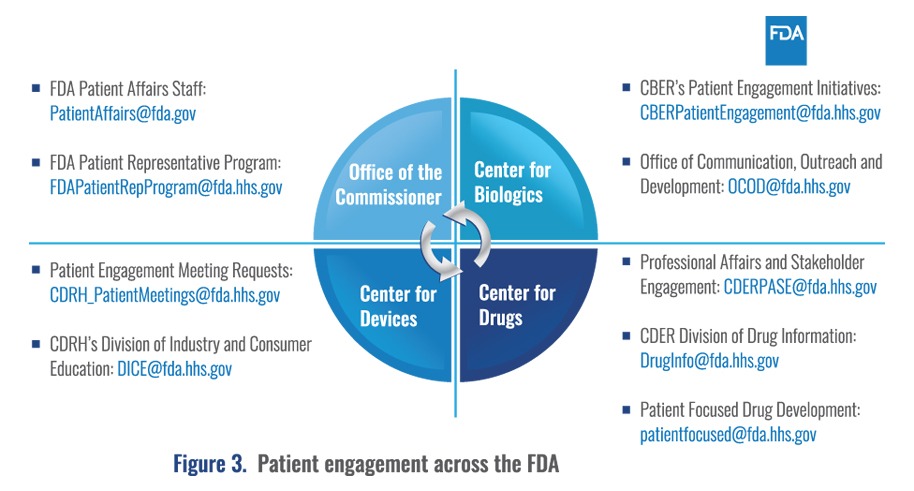
She stressed that the FDA fully recognizes that patients are experts on what it is like to live with their conditions and are “uniquely positioned to inform understanding of the therapeutic context for drug development and evaluation.”
One key patient engagement activity within the FDA is the Patient Focused Drug Development (PFDD) initiative. In total, the FDA has convened 24 PFDD meetings on specific disease areas, which are attended by a cross section of stakeholders, including patients, advocates, researchers, drug developers, and healthcare professionals. The goal of these sessions is to listen to patients’ perspectives on their disease, symptoms, and treatment options. A key learning from these meetings is that many patients want to be as active as possible in the work to develop and evaluate new treatments. FDA also actively participates in PFDD meetings led by patient organizations focusing on many disease areas.
She encouraged anyone who hasn’t attended a PFDD meeting to try to do so as it provides a valuable insight into a key way in which the FDA engages patients in the drug development process. The meetings can be attended remotely, and summaries are available on the FDA website.
Diane spoke passionately about the commitment of everyone in the FDA to put patients at the heart of everything they do. She said, “We do what we do because we want safe and effective products for patients, and we want them available in a timely way.”
Peyton did not mince her words about why it is so important for the biopharmaceutical industry to drive a customer-centric approach to drug development.
“Even before the impact of COVID-19, there was real urgency to make clinical trials more patient centric. There are currently 40,000 clinical trials recruiting in the US, and 80% are delayed due to recruitment problems. 85% of clinical trials fail to retain enough patients.”
In her presentation, Peyton focused on Patient Centric Protocol Optimization (PCPO), which is one of the key areas in which her organization has seen encouraging results. PCPO is a framework to solicit feedback from patients and study sites to ensure that patient needs are met. This approach can include patient surveys, web listening, and patient burden analysis. A case study on PCPO was included in the related MAPS workshop presentation presented by Jamie Kistler and Tricia Gooljarsingh.
“This process has revealed that a lot of previous assumptions were wrong. We used to obtain feedback from site nurses as opposed to patients themselves, and our assumptions around patient burden and preferences were often incorrect.”
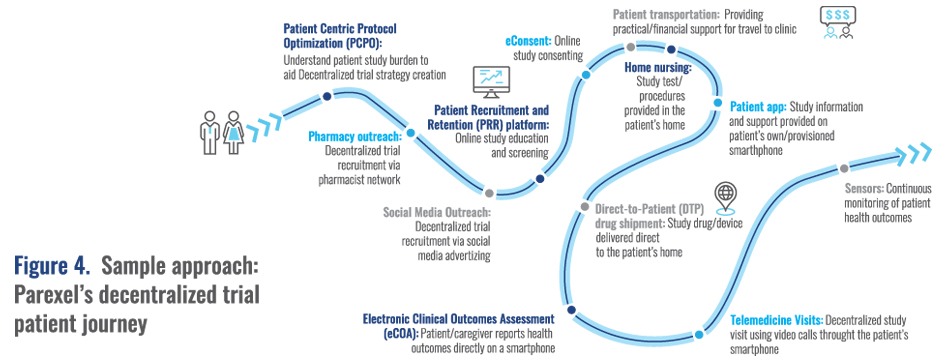
She also emphasized the increasing importance of decentralized trials and shared an example of a decentralized trial patient journey (Figure 4). While every journey is different depending on the patient and the therapy area, what is similar is the need to start engaging with patients early. She also highlighted the importance of using mobile options and other technology in decentralized trials. COVID-19 has been a catalyst to support innovation in the application of decentralized clinical trial tactics such as telemedicine visits and home nursing.
Looking ahead, she urged that there is still a lot more that can be done and asked everyone in the room to have a voice. “This community can really play a key role in driving forward the innovations to make trials more patient centric.”
Looking ahead, Peyton is excited about the following opportunities:
- Patient advisory councils
- Mock trial visits
- Patient concierge (for example helping with transport or reimbursement issues, which can really impact a patient’s ability to stay in a study)
- Patient communities (especially after the trial has finished, which can also provide valuable real-world evidence data)
- Digital ethnography research (the study of people in a real-world environment where researchers collect behavior data from participants either in a mobile or online environment)
- Alternative care sites
As the final speaker, Roz emphasized that in this multidisciplinary collaboration, Medical Affairs is uniquely positioned to drive organizational change where patient centricity resides at the core. “We can all embrace the concept of patients as one of our most critical stakeholders, but that doesn’t mean people see its relevance to what they do every day. To advance a cultural shift, it needs to start with the organization’s ‘why,’ and this needs to align with the different functional groups.”
She recommended creating “sharing platforms” where information and insights from the various patient-related activities taking place across an organization are shared and utilized to inform the overall patient engagement strategy. She urges that this intelligence should not just come from thought leaders and healthcare providers but from a wider network, including umbrella organizations, industry coalitions, health authorities, etc. The “cycle of learning” is further supported by demonstration projects so key learnings can be shared with these external networks in a non-competitive way.
Roz stressed the importance of leadership endorsement saying, “It won’t happen unless you have significant leadership endorsement at every level across the company. If you don’t have that endorsement, patient engagement will be the first thing that’s dropped, so you need to ensure what you are doing is core to the business.”
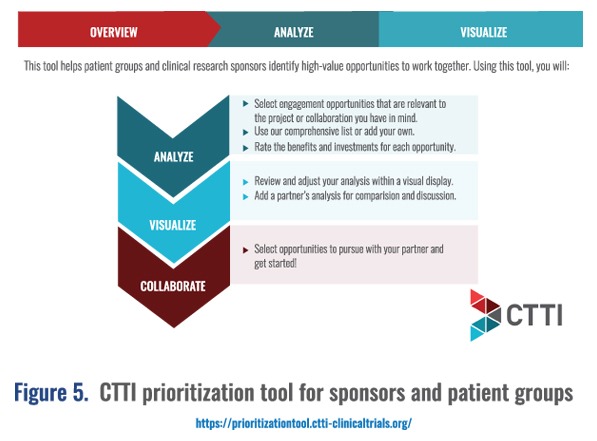
Concluding on metrics, she referenced some important tools that can be used by Medical Affairs professionals and cross-functional partners (these are included along with other key resources in the related MAPS Annual Meeting workshop available on-demand at https://community.medicalaffairs.org/on-demand-conferences):
Concluding the plenary session, Dr Len Valentino noted, “Having listened to the presentations today, what comes across loud and clear is the importance of understanding the patient journey and involving the patient early in the process. Medical Affairs can create the narrative that tells the story of the patient that allows all the other functions to do their jobs better by focusing on what is important to the patient.”
Acknowledgements
The authors wish to acknowledge Tricia Gooljarsingh, PhD (Momenta Pharmaceuticals), Jamie L. Kistler, PhD (Parexel International), and Isabelle Bocher-Pianka (Ipsen) for their contributions to the concept, design, and content development for the MAPS Annual Meeting patient centricity plenary session and workshop and this accompanying Elevate article. They would also like to recognize the significant effort made to ensure that those unable to attend in person due to COVID-19 travel restrictions could participate remotely and engage in discussions. Editorial support for this article was provided by Patricia Barnfather (Barnfather Communications, Ltd) and Diane Neer (Parexel International).
Reference
- CISCRP. (2019). 2019 Perceptions & Insights Study. Boston: Center for Information and Study on Clinical Research Participation.
Download the Full Article



















 In short – if you don’t trust someone enough to hire them remotely, you shouldn’t trust them to work for your organization in any capacity. Epidemiologists from every corner of the world are hiring right now (in dozens of different languages) to make new discoveries via remote technologies like Zoom. Your team can do the same. They just need to know how.
In short – if you don’t trust someone enough to hire them remotely, you shouldn’t trust them to work for your organization in any capacity. Epidemiologists from every corner of the world are hiring right now (in dozens of different languages) to make new discoveries via remote technologies like Zoom. Your team can do the same. They just need to know how. Video chats should be the preferred medium for your hiring process. Webex, Skype, Zoom, and Google Meet all do the same thing. Choose whatever tool you believe is easiest to navigate and understand. Whatever you select – consistency matters for new recruits and employers alike. Don’t alternate between platforms.
Video chats should be the preferred medium for your hiring process. Webex, Skype, Zoom, and Google Meet all do the same thing. Choose whatever tool you believe is easiest to navigate and understand. Whatever you select – consistency matters for new recruits and employers alike. Don’t alternate between platforms.