PODCAST: Expert Engagement — Driving the Strategic Impact of Medical Affairs
MAPS in conversation with Robert Groebel, VP Global Strategy, MONOCL, and Danie du Plessis, VP Medical Affaris, Kyowa Kirin
MAPS in conversation with Robert Groebel, VP Global Strategy, MONOCL, and Danie du Plessis, VP Medical Affaris, Kyowa Kirin
Eileen Sawyer didn’t always intend to work in pharma—in fact, she hadn’t ever planned to have a career in science.
Growing up in a Boston suburb, neither of her parents worked in the sciences. Her dad was a computer programmer and her mom edited college textbooks. But the family was always passionate about the natural world. Sawyer recalls spending family vacations at national parks, identifying plants and animals together. She’s a self-proclaimed science nerd. She loves the details and especially translating those details to application. Quality science is where it all begins.
In high school, she says she was a ‘big reader,’ and planned to become a writer. In college, that idea changed when she developed an interest in psychology. She loved learning how people think, and she especially liked studying the connection between psychology and biology.

Eileen Sawyer, Vice President, Global Medical Affairs, UniQure
As part of her studies, Sawyer took an introduction to a neuroscience class—and fell in love with it. She enjoyed combining hard science with an understanding of psychology to discover why people are the way they are. It had a storytelling flavor that appealed to the writer in her.
She started working towards her Bachelor of Arts in Neuroscience, and spent her summers interning in labs that studied animal behaviour. Her new career goal became discovering connections between the brain and behavior that could improve healthcare, and after college, she pursued a PhD in Neuroscience research. She hoped to make scientific advancements that would contribute to development of new drugs to help people.
However, after her PhD, Sawyer began questioning if academia was the right fit. She continued on to her post-doc but felt frustrated. Their team was doing pre-clinical drug discovery, but there were stacks of studies sitting in file drawers waiting to be published. It was great science, but she longed to work more directly on creating new medicines. After all, she had gotten into science to help people. She realized that more than anything, she wanted to work closer to patients—and have a greater impact on their care.
That’s when she made the leap into pharma. Initially, she started by doing some freelance science writing and editing on the side. But the more she got to know the world of pharma, the more she realized that the best place to have the type of impact she wanted was in one of those companies itself, directly working on bringing new drugs to patients. To open up new opportunities, she tried old fashioned networking—asking people about what they do and reaching out to leaders in the industry. She got involved with associations in medical writing and communications and applied to dozens of jobs.
Months later, she got a call about an editing job. But by that point, she realized she didn’t want to just write—she wanted to work inside pharma. She declined the role and continued networking and applying.
Eventually, she got the call she was looking for—an interview at Alexion in scientific communications. The team that was hiring was preparing to launch a product for bone disease. The condition was rare, and severe. Babies were born with no mineralization in their bones and died because their chest couldn’t support breathing. Of course, the work was completely different from neuro, as it was all below the neck. Still, Sawyer knew immediately that she wanted to help.
The position at Alexion was a formative experience. Sawyer had a mentor she admired, and a role that allowed for plenty of exposure to all of medical affairs, from launch preparation to strategy. Their team worked in that crucial space between the science and the impacts on actual people. In real time, she got to witness science turning into effective medicine—medicine that made tangible impacts on patients.
She loved every minute of it.
After the drug was launched, Sawyer began looking for her next step, knowing that she wanted to find another role with the same level of impact. She found the perfect answer—working on gene therapy at uniQure.
When Sawyer started at uniQure as Director of Scientific Communications, it was small company with no Medical Affairs department. Six months into her tenure, she became the firm’s first Director of MA, and for the first two years in the role, she was a one-woman department. More recently, she has been able to build out the team and increase their impact.
Now, she leads more than seven people with different MA specialties ranging from communications and field medicine to health economics and strategy.
Sawyer’s MA team is focused on removing barriers to access to gene therapy. The barriers for patients fall into two main categories:
The health care provider or the patient either does not understand gene therapy or has misperceptions about it and how it can treat the condition. They may not even know a drug is in development. The answer to this challenge? Education.
Justifiably, patients and doctors have questions about the treatment. The MA team needs to work to understand those questions, bring them back inside to find answers, and then take those solutions back to the patients. Sometimes, this process requires supplemental studies or health outcomes research.
Sawyer saw a clear key to removing those barriers—listening to patients and physicians. To ensure they were receiving adequate feedback on their work, Sawyer and her team started early, reaching out to patients and physicians while publishing and announcing results from the phase 1 study.
As it turned out, listening to patients changed the team’s entire development program. From the conversations, they learned that the community wanted something completely different from the gene therapy than was expected. So, they went back to the drawing board, totally changing the design of the program.
Sawyer was responsible for presenting the change to thought leaders, MDs and top patient advocates. When she revealed the news at a small scientific meeting after the company announcement, there was an audible gasp in the room. The leaders didn’t think regulators would permit this type of change. However, when she heard from the community that provided the feedback, Sawyer was reassured that they had made the right decision. Patients and physicians were so pleased to see that the company responded to their comments, and it completely transformed their attitude towards partnering with uniQure.
“Our goal was to deliver what they need,” says Sawyer. “They saw that.”
Now, uniQure has patients provide input into everything from their protocol to their patient materials and scientific steering committees. Again, and again, Sawyer has seen them contribute remarkable insights the MDs hadn’t thought about. She recalls one patient at a steering committee who pointed out an issue with the wording in a particular passage. It mentioned barrier protection during sexual contact, and he asked, “How are you defining sex? How inclusive are you being?” The team used his feedback to rewrite the section with more inclusiveness and clarity.

The uniQure team after defending the switch to AMT-061 at the European Medicine Agency. Sawyer is sixth from right.
“Sometimes, wearing the medical hat you forget to think about how it would play in the real world. What does it really mean for someone’s life?” she says.
Sawyer is making it her life’s work to focus on the patient—the most important stakeholder in drug development. She wants to help them take ownership over their care and make informed decisions. To do that, they need access to information—both directly and via access to educated health care providers. Her team presents scientific findings at patient meetings as well as medical meetings, and they engage with patient association leaders around science and policy. They even involve patients in economics and outcomes research. The goal is to foster true partnerships while developing each drug.
“At the end of the day, it’s about giving the patient control,” Sawyer says. “The best scenario is for a patient to have many options and be empowered to choose the best choice for them. The role of pharma in this setting is to equip them with the tools to make those decisions.”
Throughout her career, she has never lost the desire that drew her to pharma. It’s the desire to turn cool science into medicine and make something that matters—something that will make someone’s life better. For her, a great day is one where she has the chance to talk to a physician and hear a patient story. Learning about the tangible impacts that the science is making on patients is what keeps her coming back.
We had a chance to sit down with Eileen. Enjoy watching our interview to see how she responds to questions about:
This article is graciously contributed by Excellerate: Patient-Focused Engagement for Pharma.
In this second of two podcast episodes, Pete Piliero moderates a discussion on contributions Medical Affairs makes to Asset Strategic Plans and Resourcing of a Medical Affairs Plan.
MAPS speaks with Beat Sümegi, SVP Medical with Sanofi, about major areas of change and the need to help Medical Affairs professionals build new skills to succeed in this world of increased responsibility and opportunity.
This Webinar features Medical Affairs and leading technology thought leaders exploring emerging trends and viewpoints on the next frontier for digital in Medical Affairs and the broader healthcare environment with the “New Normal”.
Pete Piliero, MD, moderates a discussion on contributions Medical Affairs makes to Asset Strategic Plans.
Download the full article here
By: Kirtida Pandya1, Jacqueline Waldrop2, Maria E. Vassilakis3, Marc Sirockman4, Deirdre Jordan5, Ogün Sazova6, Patricia Jassak7, Tim Mikhelashvili8, Sarah Funderburk9, Ivan Desviat10
In the Medical Affairs Professional Society (MAPS) community, external medical education can be categorized based on the extent of influence and industry involvement; however, it should always have the ultimate goal of optimizing patient care and improving health outcomes. Medical Affairs (MA) professionals take the lead to reach this goal by establishing external education programs to address knowledge, competency, or performance gaps. Educational strategies are implemented while fully complying with all applicable laws and codes and considering variables such as regional differences. External medical education demands are continuously evolving, and MA needs to continue to evolve with these changes to establish effective education strategies. Furthermore, the swift change to virtual education platforms due to the current COVID-19 pandemic requires speed and agility across stakeholders. The adaptation of medical education tactics will ensure effective healthcare professional (HCP) education, which will ultimately lead to improvement of patients’ healthcare.
The central mission of external medical education is to provide unbiased education to enhance healthcare professionals’ (HCP) knowledge, skills and competencies to improve patient outcomes. But what role do Medical Affairs (MA) professionals have in its creation and dissemination? The following article provides a brief overview of external medical education and gives insight into the integral role of MA professionals.
External medical education can be defined as diverse educational approaches provided to various stakeholders, such as HCPs, payers, patients, and caregivers, through education initiatives that aim to address identified knowledge, competency or performance gaps. Medical education may address therapy area and disease education gaps through scientific conferences, company-led education, continuing medical education (CME) grants and fellowships or diverse collaboration with scientific and patient advocacy societies (https://community.medicalaffairs.org/on-demand-conferences, The Role of Medical Affairs in External Medical Education: A Roadmap, Slide 11).
In its simplest terms, the primary goal that unites the entire industry across its MA functions is better patient care. In a systematic literature review of 39 studies, Marinopoulos et al. showed that independent medical education was indeed effective in improving the clinical outcomes of patients1. This was supported by Cervero and Gaines, who found that CME improves physician performance and clinical outcomes2.
MA professionals play a major role in the education needed to improve physician performance and patient outcomes. Based on their insights from diverse channels, they identify knowledge and competency gaps, and unmet medical needs. These can be addressed and translated into education for all stakeholders. MA professionals communicate and disseminate fair-balanced information that is important in guiding relevant strategies or tactics; however, their exact role varies depending on the level of independence of the approach and the extent of direct involvement, policies, and compliance requirements.
Independent medical education is supported through grant funding and is planned and implemented without the influence or control of the commercial supporter. Programs can be either funded through unsolicited grants, where third parties submit proposals, or through grants submitted in response to a Call for Grant Applications (CGA) or Request for Proposals (RFP). A wide variety of educational strategies are used at different lifecycle stages of a medicine or device. In the earlier stages it is crucial to assess the needs of healthcare providers and begin to close knowledge gaps, while the peri- and post-launch stages require predisposing, enabling and re-enforcing educational methods (Figure 1)3.
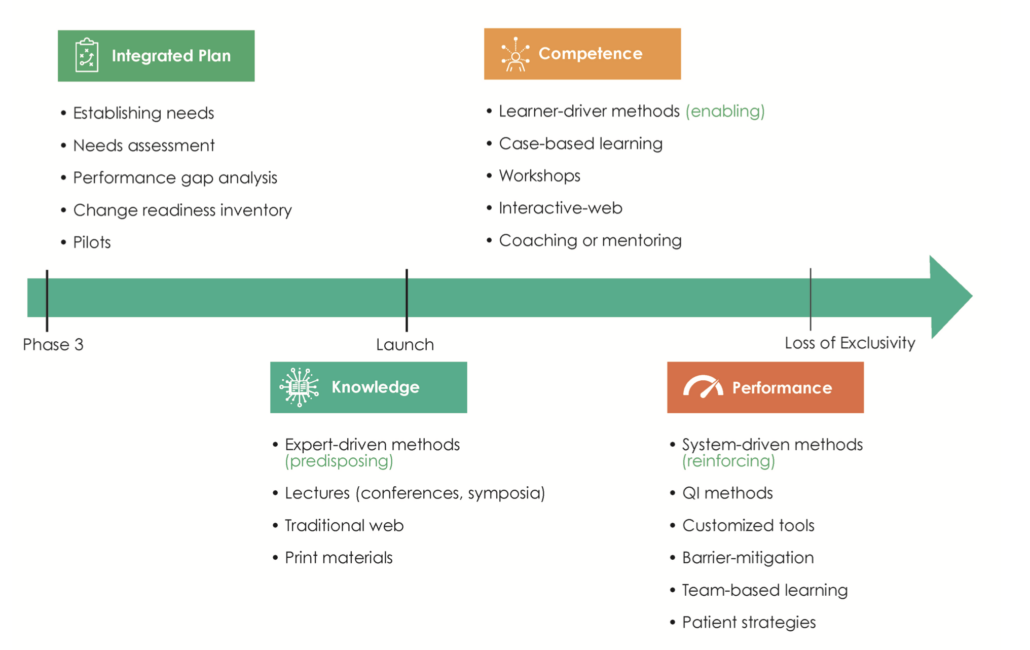
Figure 1. The planning of external medical education is aligned to the life cycle stages of a medicine. QI, Quality Improvement.
To strive for success, MA professionals are advised to keep the end goal in mind, thereby following a “backward planning” strategy. The final ideal state is identified first, then potential knowledge and competency gaps are identified, and finally, budget is allocated to a grantmaking strategy. MA professionals must also take into account the therapeutic landscape and other compounds/products in the specified area of interest.
Unsolicited independent medical education grants have historically utilized a reactive transaction where companies accept unsolicited proposals from accredited providers. MA professionals have had to change their approach to be able to proactively influence the healthcare landscape across a broad spectrum of stakeholders’ perspectives. Supporting needs assessment projects and extensive information-gathering must be used first to inform an effective independent medical education strategy, whether grants are submitted spontaneously or in response to a CGA or RFP.
Company-led medical education is another widely used form of external medical education. These activities are organized by individual pharmaceutical companies and might involve scientific committees, and/or independent scientific and professional organizations. Examples include scientific symposia, patient educational programs, company-sponsored meetings, and educational websites, among others.
Like independent medical education, company-led education is bound to the highest standards for quality, transparency and ethics in medical learning. Content must be relevant, credible, and timely, addressing educational gaps through a sound instructional design and outcome measure plan.
Regulatory agencies have diverse views on the classification of medical education developed by the pharmaceutical/biotech/device industry. Medical education and educational materials are rarely defined by their intent, but by the originator or the supporter. In this regard, industry-developed education/educational materials are considered promotional in many markets regardless of their nature and the internal function that develops them.
Medical education may play a role in influencing the market growth of therapeutics by increasing the awareness of disease states, treatments, and changing guidelines. However, the overall intent of medical education must not be to promote company products, devices, or solutions, but to improve HCPs’ knowledge of relevant data and integrate this into clinical competencies and skills which optimize patient outcomes.
It is important to note that various functions within the industry develop educational materials and scientific programs. While the medical education developed by the MA function does so in a scientific, non-promotional manner, there are components of education regularly developed or used by industry’s commercial function to complement their solutions with a primary intent to increase market share and sales of a product.
Legal and compliance implications in defining and implementing company-led education are extensive and are not addressed in this article. The MAPS Focus Area Working Group (FAWG) on External Education will be addressing these topics in an upcoming Standards and Guidance document and future e-Learning modules.
MA professionals need to measure the impact of educational initiatives on clinical practice and patient outcomes. Assessing relevance and effectiveness should be a continuous process throughout planning and implementation of an activity and needs assessment insights should inform the outcomes assessment plan. Activities should be continuously assessed for relevance and effectiveness and modified when needed. Moore and colleagues proposed a model of outcomes assessment that can aid MA professionals when evaluating activities for their impact on HCP performance and patient outcomes4.
To achieve positive outcomes for patients and healthcare systems, MA professionals need to be aware of potential hurdles they may encounter. Not only do they have the responsibility to ensure delivery of high-quality medical education externally, but they must also find a way to demonstrate the importance and positive impact of medical education to internal stakeholders. Furthermore, where previously physicians had the lead in making treatment decisions, due to rising healthcare costs, decision-making power is gradually shifting to a new set of stakeholders e.g., nurse practitioners and physician assistants who are helping to drive cost containment. It is crucial to be aware of regional differences affecting external medical education tactics (Figure 2). Variations in standard of care, availability of therapeutic products, and region-specific regulatory processes must be acknowledged when designing educational programs.
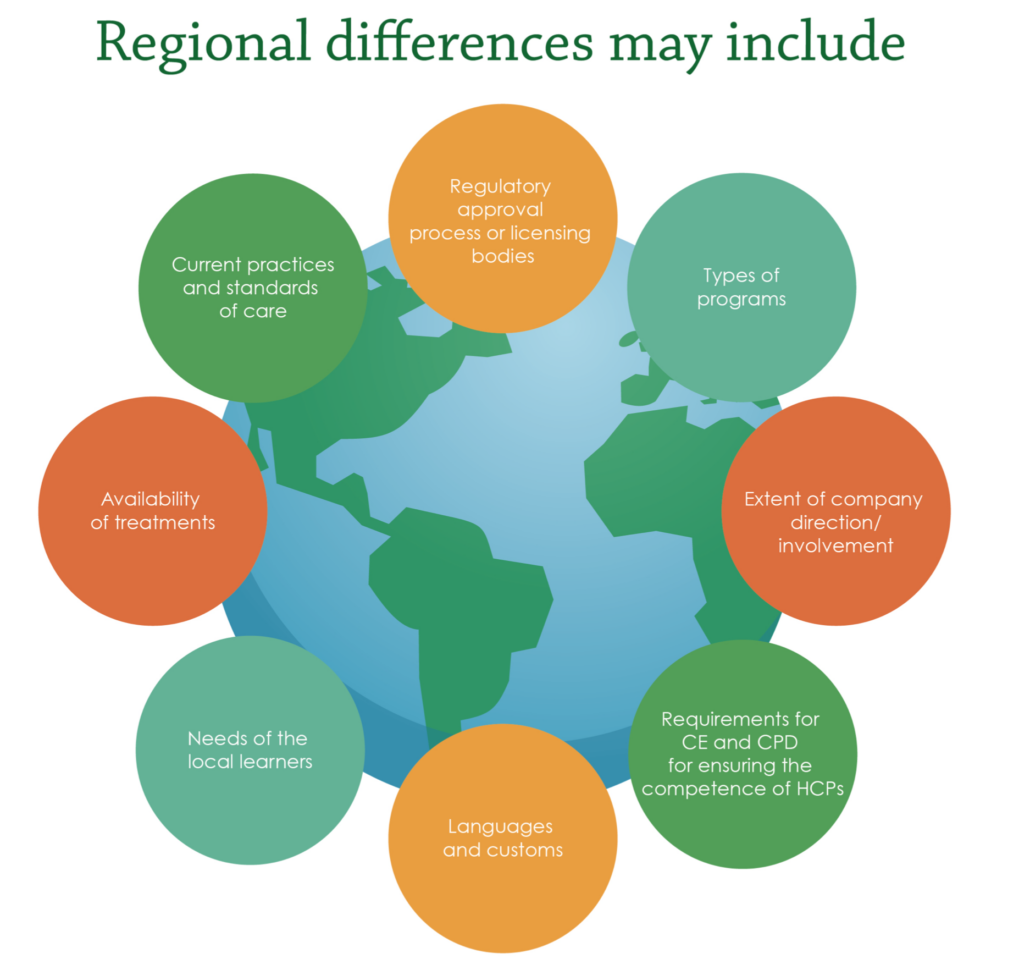
Figure 2. Regional differences in external medical education. CE, Continuing Education; CPD, Continuing Professional Development; HCPs, Healthcare Professionals.
External medical education is rapidly evolving, and MA professionals must be ready for swift changes. Healthcare scientific data continue to increase and originate from an ever-expanding range of sources such as real-world evidence and social-listening programs. The need for external education supporting HCPs as they decipher and incorporate this information requires adaptive education strategies. Medical education is part of HCPs’ lifelong professional development and must address learning needs most relevant to their daily practice5. Increasing transparency in the industry requires data generation and dissemination around external medical education programs, particularly transfers of value (TOV) to HCPs. Additionally, patient advocacy groups are more frequently included in decision-making, maximizing patient insights and directing education strategies towards a more patient-oriented approach.
The recent rapid transition to virtual learning has enhanced disseminating educational content during the ongoing COVID-19 pandemic. MA professionals must adapt to challenging times and find new, diverse ways to generate, and disseminate educational content effectively to support HCPs’ and patients’ needs. A “one size fits all” approach is inadequate for virtual content when delivering medical education in the current situation. A tailored, agile approach aligned to the appropriate setting and audience will allow MA professionals to provide successful digital education and establish the most suitable forum within which to improve timely data dissemination. The right information and education delivered in the appropriate format to HCPs will translate into enhanced and more effective patient care, ultimately improving patient health outcomes.
MA professionals are uniquely positioned to serve as factual, impartial, trusted partners who align company strategy to meet healthcare stakeholders’ and patients’ needs. They lead implementation and support of external education with the goal of improving patient outcomes. Gathering internal and external stakeholder insights while keeping the end goal in mind will transform the ever-changing relevance and value of MA strategy and tactics. Thus, as the field of external medical education evolves, MA professionals must adapt accordingly by keeping abreast of the latest healthcare institutional system changes, regulatory codes and compliance needs, regional differences, and technology advances. This will ensure that our key stakeholders (HCPs) have access to quality, evidence-based scientific data to address education gaps to optimize patient care and impact health outcomes.

This MAPS webinar presents an overview of the current and near term Digital Therapeutics landscape and details real-world applications of Digital Therapeutics to illustrate potential in driving transformation of clinical practices to improve patient outcomes.
OBJECTIVES: Identify the major trends and drivers towards digital transformation and drive Medical Affairs towards a digital future.
Download the Full Article Here
By João D. Dias1, Cesar Sanz Rodriguez2, Raphael T.B. Tan3
1 Medical Affairs & Clinical Development, Haemonetics S.A., Switzerland, Medical Affairs Professional Society (MAPS) MedTech Focus Area Working Group (FAWG) EMEA Co-Lead
2 Medical Affairs, MSD International GmbH, Switzerland, MAPS MedTech FAWG EMEA Co-Lead
3 International Medical Executive Consultants (IMEC), The Netherlands, MAPS MedTech FAWG member

This article provides an overview of the new MedTech European regulatory environment and opportunities for the Medical Affairs function to evolve and bring value to the respective organizations. The European Regulations ask for an increased effort from manufacturers to generate and communicate clinical evidence on the safety and performance of their Medical devices, In vitro-diagnostics and Drug Device Combinations. In conjunction with increased quality standards they make a compelling case for Medical & Scientific Governance with a prominent role for Medical Affairs in many pre- and post-market processes. A transformation of Medical Affairs into a strategic business function makes the medical device industry an exciting place to be for Medical Affairs professionals.
For over two decades, Medical devices and In-vitro Diagnostics have been regulated in Europe by Directives for MEDICAL DEVICES (MDD) and IN VITRO DIAGNOSTICS (IVDD)1,2,3 that were published in 1993 and 1998 respectively. A separate Directive for Active Implantable Medical Devices (AIMD) was published in 1990 with a last revision in 2009. According to these Directives, devices are approved for the European Single Market only after having obtained CE Mark for which manufacturers need to demonstrate conformity to essential requirements relating to the device’s performance and safety for patients and users. After public consultation by the European Commission in 2008 it became clear that an update of the Directives was needed, one reason being the simple fact that new technologies such as companion diagnostic devices were not yet covered. The need for revision gained traction after incidents with breast implants, transvaginal meshes around 2009 and metal-on-metal hip prostheses a couple of years later. Eventually, the revision process that started in 2012 resulted in the Medical Device Regulation (MDR) in which MDD and AIMD were combined and the In Vitro Diagnostics Regulation (IVDR)4,5. The new Regulations were published in 2017, with May 25, 2017 as the official date of entering into force. A transition period to full implementation of the MDR and IVDR was allowed for three and five years respectively, which means the MDR applies from May 26, 2020 and IVDR from May 26, 2022.
So far so good… and then the COVID-19 crisis struck Europe, right at the moment when the medical device industry and notified bodies are transitioning to the new Regulations. Therefore, in order to “take the pressure off national authorities, notified bodies, manufacturers and other actors so they can focus fully on urgent priorities related to the coronavirus,” the European Commission has decided to move back the date on which the new MDR would fully apply by one year, to 26 May 2021. MedTech Europe, the European trade organization of medical device manufacturers, has advocated for a similar delay for the IVDR.
Since the early days of the global COVID-19 crisis the medical industry has made a so far unseen effort in finding (bio-)pharmaceutical solutions, developing vaccines and reliable test kits. Simultaneously, in an attempt to address the relative shortage of masks and intensive care equipment such as ventilators, traditional medical device manufacturers ramped up production. Although regulators accommodate the surge of new devices by fast tracks and exemption rules, new devices are subject to meticulous assessments of performance and safety. And rightly so, since national policies to curb transmission of the virus rely on the quality of diagnostics and personal protection equipment. Especially important is the scrutiny in assessing new medical devices which are intended to be used in the management of the most vulnerable and severely ill COVID-19 patients who end up in hospitals and ICUs.

In general the new Regulations devices, improve traceability and transparency and define stricter requirements to clinical evidence and post-market surveillance. The MDR now also regulates devices for cosmetic purposes such as colored contact lenses and cosmetic implant devices. The introduction of Unique Device Identification should improve traceability, and transparency is created by the European Databank of Medical Devices (EUDAMED) where all mandatory regulatory documentation on each device is kept and updated. Manufacturers must re-certify devices in accordance with the new regulations and update their technical documentation accordingly with special attention to higher clinical requirements for class III and implantable devices. A shift of focus from a mere pre-market perspective towards a life-cycle approach also includes stricter requirements regarding post-market surveillance, post-market clinical follow-up and vigilance.
This brings us to the Clinical Evaluation, which lies at the basis of CE mark approval and the life-cycle approach. It is defined as “a systematic and planned process to continuously generate, collect, analyze and assess the clinical data pertaining to a device in order to verify the safety performance, clinical benefits of the device when used as intended by the manufacturer.” The Clinical Evaluation process is described in a MEDDEV (MEDical DEVices) guidance document. MEDDEVs promote a common approach to be followed by manufacturers and notified bodies that are involved in conformity assessment procedures. Although these MEDDEVs are not legally binding, it is expected that their guidance be followed, ensuring the uniform application of the various elements of the directives/regulations. MEDDEV 2.7/1 rev. 46 is the guidance document with regards to Clinical Evaluation and is prescriptive on the process, the required qualification of the evaluators and the contents of a Clinical Evaluation Report (CER).
The CER should “describe the intended clinical benefit and provide evidence of safety and performance” where performance is defined as “the ability of a device to achieve its intended purpose as stated by the manufacturer.” This report describes the risk profile of the device based on the technical documentation and provides an appraisal of all available clinical data related to safety and performance. Any evidence gaps and residual risk need to be addressed in Post-Market Clinical Follow Up (PMCF) studies to demonstrate long-term performance safety. The results from the intensified surveillance are laid down in the Periodic Safety Update Report (PSUR, mandatory for Class IIa/b and III) and Summary of Safety and Clinical Performance (SSCP, for implantables and Class III). These documents must be uploaded in EUDAMED, which allows public access to the SSCP.
Although European market approval of new devices can still be obtained by referring to clinical data of predicate equivalent devices, the MDR explicitly lists criteria by which equivalence can be claimed from a technical, biological and clinical perspective. The equivalence under the MDD was less well defined. Under the MDR, the predicate device must have a similar design, use the same materials and come in contact with the same tissues and body fluids, and be used for the same clinical indications. If a device does not meet these criteria, manufacturers need to generate their own clinical evidence with appropriate clinical investigations.
CE Marking requires Notified Body involvement for most medical device classes and is related to implementation of a Quality Management System, which must include plans pertaining to Clinical Evaluation, Post-Market Surveillance (PMS) and Post Market Clinical Follow-up (PMCF). For non-sterile Class I devices, manufacturers can conduct the conformity assessment themselves and basically self-certify. For Class I devices that are sterile, measuring or reusable surgical instruments as well as for all higher device classes, oversight of a Notified Body is required. In the end, it is the Notified Body that issues a CE Marking Certificate and ISO 13485 Certification. Only with these certificates in place the manufacturer can prepare a Declaration of Conformity and put the CE Mark sign on their products and labelling.

In Vitro diagnostics (IVDs) are medical devices with which tests are performed using human specimens such as urine or blood. Familiar examples of such devices are pregnancy tests and tests for determining the level of glucose or cholesterol in the blood. The current directives distinguish between medium risk and high-risk lists of IVDs. Those IVDs not captured in these lists are automatically classified as low risk. Obviously more stringent market authorization procedures with Notified Body oversight apply to high risk IVDs. Whereas in the past only a minority of IVDs required involvement of a Notified Body, under the new classification an estimated 90% will now require it. The IVDD had some gaps and this binary system was thought to be no longer sufficient. Hence, like for the MDR, a risk-based approach classification was introduced based on the severity of the disorder tested for and possible consequences of an incorrect test result. Instead of two lists, the new IVDR now distinguishes four categories, Class A (lowest risk), Class B, Class C, and Class D (highest risk) and dictates that Class B and above IVDs will require oversight from a Notified Body as part of their conformity assessment.
Aside from the risk-based classification, it will not surprise you that the new IVDR has more features in common with the MDR. As is the case for medical devices, the IVDR requires clinical evidence and post-market performance follow-up. This will require a Performance Evaluation plan and report for all IVD Classes, which will describe how to demonstrate scientific validity, analytic performance, and clinical performance.

Some medicines are used in combination with a medical device, usually to enable the delivery of the medicine. In the European Medicines Agency (EMA) view, if the principle intended action of the combination product is achieved by the medicine, the entire product is regulated as a medicinal product under Directive 2001/83/EC7 or Regulation (EC) No 726/20048. However, MDR’s Article 117 brought some relevant additions. First, two categories were defined: (a) Integral, where the medicinal product and the device form a single integrated product (e.g. pre-filled syringes and pens) and (b) Co-packaged, where the medicinal product and the device are separate items contained in the same pack (e.g. reusable pen for insulin cartridges). Next, Article 117 also incorporated some relevant amendments to Directive 2001/83/EC to ensure combination products comply with the medical device legislation. Per MDR’s Article 117, the marketing authorization application should include a CE certificate for the device or an opinion from a Notified Body on the conformity of the device (except for non-sterile, non-measuring and non-reusable surgical Class I devices).
Probably as a reaction to the rapid growth of combination products in recent years and the need to bring further clarity in this area, in June 2019 EMA released for public consultation a draft Guideline on Quality Requirements for Regulatory Submissions for Drug-Device Combinations9. The aim of this Guideline is to clarify expectations laid down in Directive 2001/83/EC and address the new obligations in the MDR. EMA makes it clear that the Notified Body assessment and marketing authorization review would not result in duplicate assessments. The former will review the device alone, while the latter will ensure the safety and efficacy of the drug are not compromised by the inclusion of the device part. The consultation period ended in August 2019 and EMA is now due to finalize the Guideline in the second quarter of 2020.
It is also worthwhile making a reference to medical devices which may contain an ancillary medicinal substance to support the proper functioning of the device (e.g. drug-eluting stents). These products should comply with the medical device legislation. Yet, the manufacturer should also seek a scientific opinion from EMA on the quality and safety of the ancillary substance if it is derived from human blood or human plasma, or if it is within the scope of the centralized procedure for the authorization of medicines. For other substances, the Notified Body can seek the opinion from EMA or a national competent authority. Of note, EMA has recently issued a Consultation Procedure for Ancillary Medicinal Substances in Medical Devices.
Companion diagnostics are seen by EMA as in vitro diagnostic tests that support the safe and effective use of a specific medicinal product, by identifying patients that are suitable or unsuitable for treatment. Applicable regulations were discussed above. Yet, before the Notified Body can issue CE Marking, it must seek a scientific opinion on the suitability of the companion diagnostic to the medicinal product concerned from EMA or a national competent authority, as appropriate. Similarly, a scientific opinion would also be needed for some other medical devices made of substances that are absorbed by the human body to achieve their intended purpose. These devices are normally introduced into the human body via an orifice or applied to the skin. Last, we should not forget the so called “borderline products”. These are complex healthcare products for which there is uncertainty over which regulatory framework applies. Common borderlines are between medicinal products, medical devices, cosmetics, biocidal products, herbal medicines and food supplements. The European Commission publishes the ‘Manual on borderline and classification in the Community regulatory framework for medical devices’ which provides examples and recommendations for determination of classifications. National competent authorities classify borderline products either as medicinal products or, for example, as medical devices on a case-by-case basis based on the product’s composition and constituents, its mode of action and its intended purpose. This determines the applicable regulatory framework.

The new European regulatory environment (MDR, IVDR and EMA guidance on Drug Device Combinations) in conjunction with expanded quality standards for MedTech products and a rapidly changing reimbursement landscape are intensifying the need for a Medical Affairs role in the product lifecycle management. Furthermore, the increased trade organization’s guidelines as well as a more complex competitive environment in which the direct comparator might not be another MedTech product but instead a Drug or a Drug Device Combinations, all work favorably for Medical Affairs to step up its game and demonstrate its value in many regards.
The traditional competencies of Medical Affairs are still required to produce the new mandatory regulatory deliverables (Clinical/Performance Evaluation Report, Periodic Safety Update Report, Summary of Safety and Clinical Performance). However, more than ever Medical Affairs involvement needs to be formalized for various other key processes ranging from product development to device application. In order to give real meaning to patient and customer centricity, the inclusion of Medical Affairs contribution is indispensable with regard to, for instance, risk analyses, claims development and identifying user training needs.
In addition, medical and clinical research functions must lead in the development of coherent and affordable clinical research programs that serve regulatory compliance, reimbursement and commercial adoption purposes. The increased effort the companies must make to generate clinical evidence will undoubtedly put strain on human and financial resources. The careful planning and design of studies should avoid waste in time and money while providing the evidence the business needs in a timely fashion. Therefore, as non-inferiority assessments are giving space to superiority assessments, an increased is expected in reliance for regulatory purposes on less conventional evidence generation strategies such as registries, collaborative research and investigator lead studies.
Furthermore, ensuring the safe application of current devices, medical communication and interactions with healthcare stakeholders and health authorities, collecting and weighing medical intelligence on future directions in healthcare all require medical scientific oversight. The medical scientific oversight will be fundamental in the advancement of the company’s innovation agenda as the bar is higher than ever, as are the associated entry and maintenance costs.

The new environment makes a compelling case for installing a structure for Medical and Scientific Governance and a proactive strategic role for Medical Affairs. This in turn opens the discussion on how to develop and organize competencies around organizational capabilities that relate to the development of innovative and relevant devices, the substantiation of medical-clinical and health economic claims and ultimately oversight to ensure safe and appropriate use. Thus, we argue that now is the time to engage in captivating thought exercises about whether a company’s fabric with a prominent role of Medical Affairs adds to the organizational capability and resilience to handle current and future challenges.
This publication represents the consensus opinions of the authors and various members of MAPS, but does not represent formal endorsement of conclusions by their organizations
1. EU Council Directive 93/42/EEC of 14 June 1993 concerning medical devices. EUR-Lex; an official website of the European Union. Published July 12, 1993. Accessed August 27 2020.
2. EU Council Directive 98/79/EC concerning in-vitro diagnostic medical devices. EUR-Lex. Published December 7, 1998. Accessed August 27, 2020.
3. EU Council Directive 90/385/EEC of 20 June 1990 on the approximation of the laws of the Member States relating to active implantable medical devices, EUR-Lex, Published July 20, 1990. Accessed August 27, 2020
4. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices. EUR-Lex. Published May 5, 2017. Accessed Aug 27, 2020.
5. Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices. EUR-Lex. Published May 5, 2017. Accessed Aug 27, 2020.
6. European Commission. MEDDEV 2.7/1 rev. 4 – Clinical Evaluation: A guide for manufacturers and notified bodies. Website of the European Commission. Released June 2016. Accessed August 27, 2020.
7. EU Council Directive 2001/83/EC of 6 November 2001 on the Community code relating to medicinal products for human use. EUR-Lex. Published Nov 28, 2001. Accessed Aug 27, 2020.
8. EU Regulation (EC) No 726/2004 of 31 March 2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency. EUR-Lex. Published April 30, 2004. Accessed Aug 27, 2020.
9. European Medicines Agency – draft guideline on the quality requirements for drug-device combinations. EMA Website. Published June 3, 2019. Accessed Aug 27, 2020.
602 Park Point Drive, Suite 225, Golden, CO 80401 – +1 303.495.2073
© 2025 Medical Affairs Professional Society (MAPS). All Rights Reserved Worldwide.
