Medical Affairs Improves Patient Outcomes Through External Medical Education
Download the full article here
By: Kirtida Pandya1, Jacqueline Waldrop2, Maria E. Vassilakis3, Marc Sirockman4, Deirdre Jordan5, Ogün Sazova6, Patricia Jassak7, Tim Mikhelashvili8, Sarah Funderburk9, Ivan Desviat10
- Executive Director and Head, Medical Services and Operations, Sandoz, A Novartis Division
- Director, Oncology Grant Officer, Global Medical Grants, Worldwide Medical & Safety, Pfizer Inc.
- Senior Director, Medical Affairs, Standards and Excellence, Astellas
- President, MedEvoke
- Global Medical Affairs Excellence Lead, Sandoz, A Novartis Division
- Medical Director, Napp Pharmaceuticals, UK
- Director, IME and External Medical Affairs, Astellas
- CEO and Co-Founder, Amedea Pharma
- Medical Insights Director, Caudex, a McCann Health Company
- Director Medical Education & Outcomes Excellence, Abbvie
ABSTRACT
In the Medical Affairs Professional Society (MAPS) community, external medical education can be categorized based on the extent of influence and industry involvement; however, it should always have the ultimate goal of optimizing patient care and improving health outcomes. Medical Affairs (MA) professionals take the lead to reach this goal by establishing external education programs to address knowledge, competency, or performance gaps. Educational strategies are implemented while fully complying with all applicable laws and codes and considering variables such as regional differences. External medical education demands are continuously evolving, and MA needs to continue to evolve with these changes to establish effective education strategies. Furthermore, the swift change to virtual education platforms due to the current COVID-19 pandemic requires speed and agility across stakeholders. The adaptation of medical education tactics will ensure effective healthcare professional (HCP) education, which will ultimately lead to improvement of patients’ healthcare.
INTRODUCTION
The central mission of external medical education is to provide unbiased education to enhance healthcare professionals’ (HCP) knowledge, skills and competencies to improve patient outcomes. But what role do Medical Affairs (MA) professionals have in its creation and dissemination? The following article provides a brief overview of external medical education and gives insight into the integral role of MA professionals.
WHAT IS EXTERNAL MEDICAL EDUCATION?
External medical education can be defined as diverse educational approaches provided to various stakeholders, such as HCPs, payers, patients, and caregivers, through education initiatives that aim to address identified knowledge, competency or performance gaps. Medical education may address therapy area and disease education gaps through scientific conferences, company-led education, continuing medical education (CME) grants and fellowships or diverse collaboration with scientific and patient advocacy societies (https://community.medicalaffairs.org/on-demand-conferences, The Role of Medical Affairs in External Medical Education: A Roadmap, Slide 11).
THE PATIENT FOCUS OF EXTERNAL MEDICAL EDUCATION
In its simplest terms, the primary goal that unites the entire industry across its MA functions is better patient care. In a systematic literature review of 39 studies, Marinopoulos et al. showed that independent medical education was indeed effective in improving the clinical outcomes of patients1. This was supported by Cervero and Gaines, who found that CME improves physician performance and clinical outcomes2.
MA professionals play a major role in the education needed to improve physician performance and patient outcomes. Based on their insights from diverse channels, they identify knowledge and competency gaps, and unmet medical needs. These can be addressed and translated into education for all stakeholders. MA professionals communicate and disseminate fair-balanced information that is important in guiding relevant strategies or tactics; however, their exact role varies depending on the level of independence of the approach and the extent of direct involvement, policies, and compliance requirements.
THE DIFFERENT TYPES OF EXTERNAL MEDICAL EDUCATION
INDEPENDENT MEDICAL EDUCATION
Independent medical education is supported through grant funding and is planned and implemented without the influence or control of the commercial supporter. Programs can be either funded through unsolicited grants, where third parties submit proposals, or through grants submitted in response to a Call for Grant Applications (CGA) or Request for Proposals (RFP). A wide variety of educational strategies are used at different lifecycle stages of a medicine or device. In the earlier stages it is crucial to assess the needs of healthcare providers and begin to close knowledge gaps, while the peri- and post-launch stages require predisposing, enabling and re-enforcing educational methods (Figure 1)3.
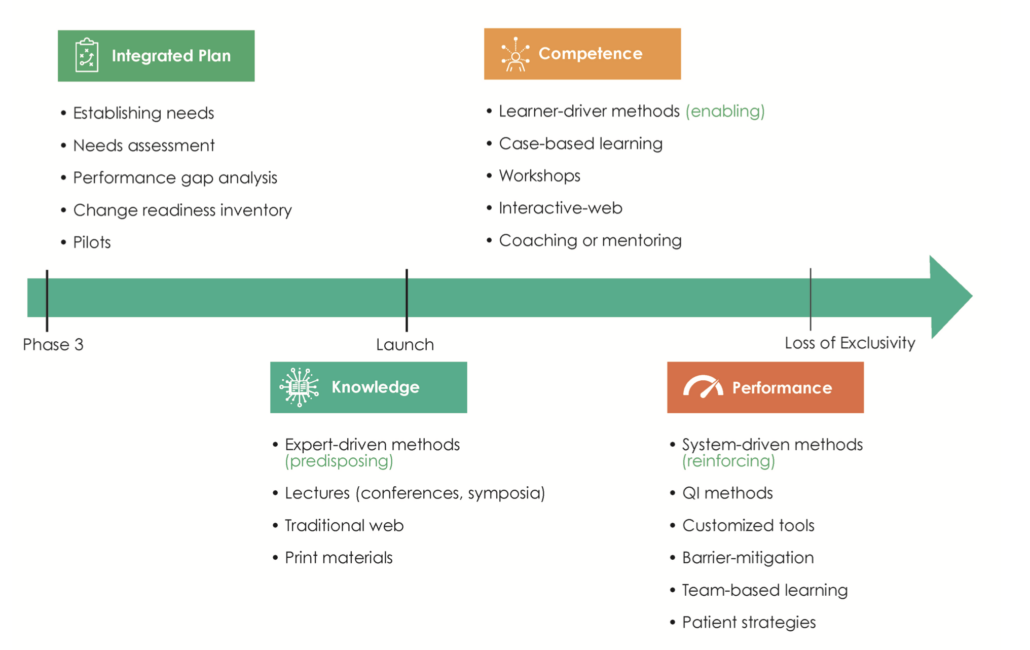
Figure 1. The planning of external medical education is aligned to the life cycle stages of a medicine. QI, Quality Improvement.
To strive for success, MA professionals are advised to keep the end goal in mind, thereby following a “backward planning” strategy. The final ideal state is identified first, then potential knowledge and competency gaps are identified, and finally, budget is allocated to a grantmaking strategy. MA professionals must also take into account the therapeutic landscape and other compounds/products in the specified area of interest.
Unsolicited independent medical education grants have historically utilized a reactive transaction where companies accept unsolicited proposals from accredited providers. MA professionals have had to change their approach to be able to proactively influence the healthcare landscape across a broad spectrum of stakeholders’ perspectives. Supporting needs assessment projects and extensive information-gathering must be used first to inform an effective independent medical education strategy, whether grants are submitted spontaneously or in response to a CGA or RFP.
COMPANY-LED MEDICAL EDUCATION
Company-led medical education is another widely used form of external medical education. These activities are organized by individual pharmaceutical companies and might involve scientific committees, and/or independent scientific and professional organizations. Examples include scientific symposia, patient educational programs, company-sponsored meetings, and educational websites, among others.
Like independent medical education, company-led education is bound to the highest standards for quality, transparency and ethics in medical learning. Content must be relevant, credible, and timely, addressing educational gaps through a sound instructional design and outcome measure plan.
Regulatory agencies have diverse views on the classification of medical education developed by the pharmaceutical/biotech/device industry. Medical education and educational materials are rarely defined by their intent, but by the originator or the supporter. In this regard, industry-developed education/educational materials are considered promotional in many markets regardless of their nature and the internal function that develops them.
Medical education may play a role in influencing the market growth of therapeutics by increasing the awareness of disease states, treatments, and changing guidelines. However, the overall intent of medical education must not be to promote company products, devices, or solutions, but to improve HCPs’ knowledge of relevant data and integrate this into clinical competencies and skills which optimize patient outcomes.
It is important to note that various functions within the industry develop educational materials and scientific programs. While the medical education developed by the MA function does so in a scientific, non-promotional manner, there are components of education regularly developed or used by industry’s commercial function to complement their solutions with a primary intent to increase market share and sales of a product.
Legal and compliance implications in defining and implementing company-led education are extensive and are not addressed in this article. The MAPS Focus Area Working Group (FAWG) on External Education will be addressing these topics in an upcoming Standards and Guidance document and future e-Learning modules.
ASSESSING OUTCOMES AND HURDLES IN EXTERNAL MEDICAL EDUCATION
MA professionals need to measure the impact of educational initiatives on clinical practice and patient outcomes. Assessing relevance and effectiveness should be a continuous process throughout planning and implementation of an activity and needs assessment insights should inform the outcomes assessment plan. Activities should be continuously assessed for relevance and effectiveness and modified when needed. Moore and colleagues proposed a model of outcomes assessment that can aid MA professionals when evaluating activities for their impact on HCP performance and patient outcomes4.
To achieve positive outcomes for patients and healthcare systems, MA professionals need to be aware of potential hurdles they may encounter. Not only do they have the responsibility to ensure delivery of high-quality medical education externally, but they must also find a way to demonstrate the importance and positive impact of medical education to internal stakeholders. Furthermore, where previously physicians had the lead in making treatment decisions, due to rising healthcare costs, decision-making power is gradually shifting to a new set of stakeholders e.g., nurse practitioners and physician assistants who are helping to drive cost containment. It is crucial to be aware of regional differences affecting external medical education tactics (Figure 2). Variations in standard of care, availability of therapeutic products, and region-specific regulatory processes must be acknowledged when designing educational programs.
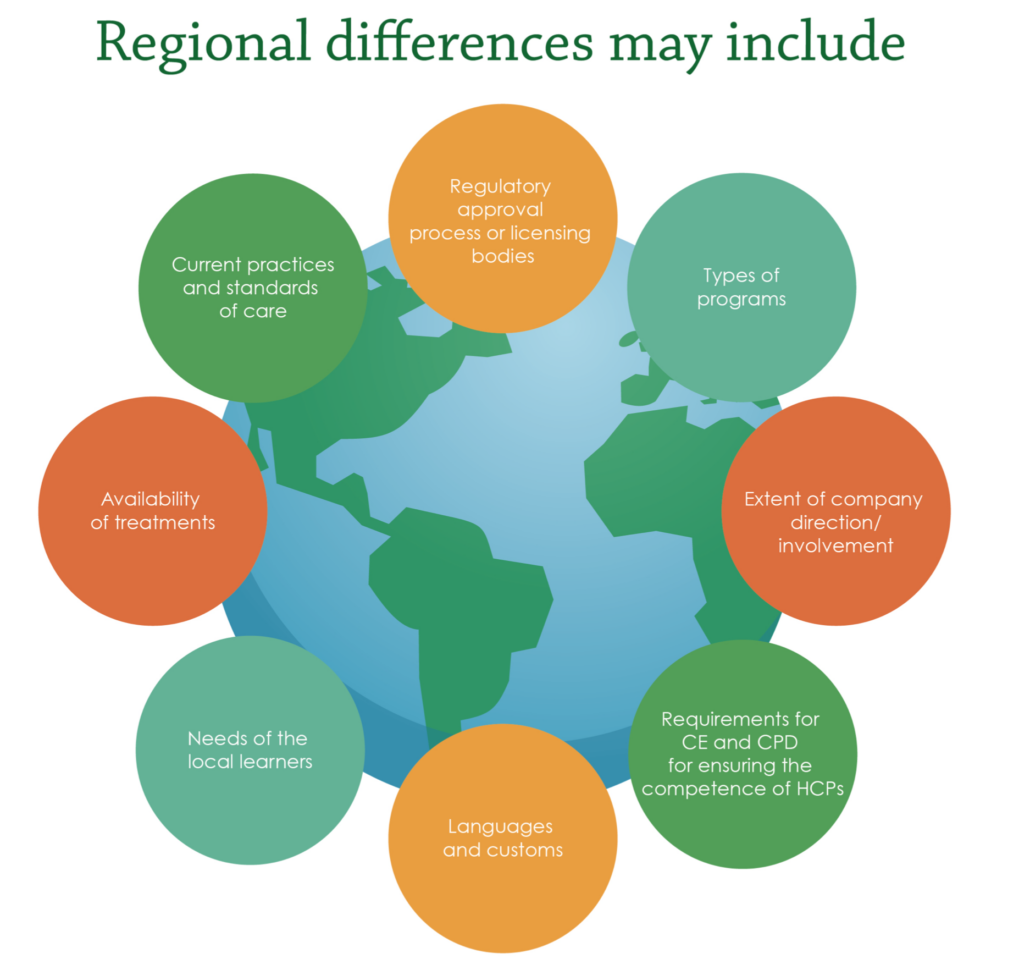
Figure 2. Regional differences in external medical education. CE, Continuing Education; CPD, Continuing Professional Development; HCPs, Healthcare Professionals.
WHY EXTERNAL MEDICAL EDUCATION NEEDS TO ADAPT
External medical education is rapidly evolving, and MA professionals must be ready for swift changes. Healthcare scientific data continue to increase and originate from an ever-expanding range of sources such as real-world evidence and social-listening programs. The need for external education supporting HCPs as they decipher and incorporate this information requires adaptive education strategies. Medical education is part of HCPs’ lifelong professional development and must address learning needs most relevant to their daily practice5. Increasing transparency in the industry requires data generation and dissemination around external medical education programs, particularly transfers of value (TOV) to HCPs. Additionally, patient advocacy groups are more frequently included in decision-making, maximizing patient insights and directing education strategies towards a more patient-oriented approach.
The recent rapid transition to virtual learning has enhanced disseminating educational content during the ongoing COVID-19 pandemic. MA professionals must adapt to challenging times and find new, diverse ways to generate, and disseminate educational content effectively to support HCPs’ and patients’ needs. A “one size fits all” approach is inadequate for virtual content when delivering medical education in the current situation. A tailored, agile approach aligned to the appropriate setting and audience will allow MA professionals to provide successful digital education and establish the most suitable forum within which to improve timely data dissemination. The right information and education delivered in the appropriate format to HCPs will translate into enhanced and more effective patient care, ultimately improving patient health outcomes.
CONCLUSION
MA professionals are uniquely positioned to serve as factual, impartial, trusted partners who align company strategy to meet healthcare stakeholders’ and patients’ needs. They lead implementation and support of external education with the goal of improving patient outcomes. Gathering internal and external stakeholder insights while keeping the end goal in mind will transform the ever-changing relevance and value of MA strategy and tactics. Thus, as the field of external medical education evolves, MA professionals must adapt accordingly by keeping abreast of the latest healthcare institutional system changes, regulatory codes and compliance needs, regional differences, and technology advances. This will ensure that our key stakeholders (HCPs) have access to quality, evidence-based scientific data to address education gaps to optimize patient care and impact health outcomes.

REFERENCES
- Marinopoulos, S. S. et al. Effectiveness of continuing medical education. Evid Rep Technol Assess (Full Rep), 1-69 (2007).
- Cervero, R. M. & Gaines, J. K. The impact of CME on physician performance and patient health outcomes: an updated synthesis of systematic reviews. J Contin Educ Health Prof 35, 131-138 (2015).
- Rogers, E. M. Diffusion of innovations (1st ed.). The Free Press of Glencoe Division of The Macmillan Co., 60 Fifth Avenue, New York 11, N. Y. (1962).
- Moore, D. E. J., Green, J. S. & Gallis, H. A. Achieving desired results and improved outcomes: Integrating planning and assessment throughout learning activities. Journal of Continuing Education in the Health Professions 29, 1-15 (2009).
- Davis, D. A., Barnes, B. E. & Fox, R. D. The Continuing Professional Development of Physicians: From Research to Practice. AMA Press (2003).






















 The amount of data we are currently generating is astronomical. According to one often-quoted article on Forbes.com, some 90 percent of the data in the world was generated over the past two years alone. [1]
The amount of data we are currently generating is astronomical. According to one often-quoted article on Forbes.com, some 90 percent of the data in the world was generated over the past two years alone. [1] “In Medical Affairs, we have a unique role because we have a deep understanding of our products and knowledge of diseases. We should be looking to fit the new knowledge and insights that we can collect from Real-World Evidence (RWE) into delivering even better solutions for patients and the healthcare system. And, because we are in daily contact with healthcare professionals, patient associations and other stakeholders, we should be the ones who play a critical role in transforming that into something that actually meets the needs and provides services and solutions for end-users. By connecting what’s coming out of our R&D, the strategy behind the products from a commercial point of view, we could build this into an overall, integrated patient care vision.”
“In Medical Affairs, we have a unique role because we have a deep understanding of our products and knowledge of diseases. We should be looking to fit the new knowledge and insights that we can collect from Real-World Evidence (RWE) into delivering even better solutions for patients and the healthcare system. And, because we are in daily contact with healthcare professionals, patient associations and other stakeholders, we should be the ones who play a critical role in transforming that into something that actually meets the needs and provides services and solutions for end-users. By connecting what’s coming out of our R&D, the strategy behind the products from a commercial point of view, we could build this into an overall, integrated patient care vision.” Dr Devoy sees scope for applying digital innovation across a range of areas but suggests that R&D, patient outcomes, and safety are the three to focus on initially. There is significant potential for how we conduct clinical trials: for example, using artificial intelligence (AI) within R&D as part of the drug discovery process, and also helping to better characterize and stratify disease and the according patient populations for study.
Dr Devoy sees scope for applying digital innovation across a range of areas but suggests that R&D, patient outcomes, and safety are the three to focus on initially. There is significant potential for how we conduct clinical trials: for example, using artificial intelligence (AI) within R&D as part of the drug discovery process, and also helping to better characterize and stratify disease and the according patient populations for study. “We read every day about how digital is transforming everything we do – be that personal finance, or interactions with retail – so I think the expectation from society is that healthcare will also transform in that way. But, clearly, healthcare has quite rightly some additional challenges relating to the sensitivity of people’s health data and taking the right care of that. So, we need to work with regulators, governments, patients and physicians to make sure that these solutions are accessible, trusted, compliant and fitted to people’s expectations.
“We read every day about how digital is transforming everything we do – be that personal finance, or interactions with retail – so I think the expectation from society is that healthcare will also transform in that way. But, clearly, healthcare has quite rightly some additional challenges relating to the sensitivity of people’s health data and taking the right care of that. So, we need to work with regulators, governments, patients and physicians to make sure that these solutions are accessible, trusted, compliant and fitted to people’s expectations. “One thing I believe we’ve done very successfully is build up an approach for actively seeking external collaboration. ”Among the initiatives is a global program called Grants4Apps, established in 2013, where Bayer reaches out to companies that are in the early stages of developing in the digital health and care space. Bayer acts as incubator and offers mentoring support and access to Bayer expertise and knowledge to help them develop their business models and solutions. Meanwhile, the Grants4Apps Dealmaker program is a unique opportunity to acquire Bayer as a customer and is tailored for mature teams, startups and companies that have a solution ready to go for identified challenges.
“One thing I believe we’ve done very successfully is build up an approach for actively seeking external collaboration. ”Among the initiatives is a global program called Grants4Apps, established in 2013, where Bayer reaches out to companies that are in the early stages of developing in the digital health and care space. Bayer acts as incubator and offers mentoring support and access to Bayer expertise and knowledge to help them develop their business models and solutions. Meanwhile, the Grants4Apps Dealmaker program is a unique opportunity to acquire Bayer as a customer and is tailored for mature teams, startups and companies that have a solution ready to go for identified challenges.