Connect with Us
602 Park Point Drive, Suite 225, Golden, CO 80401 – +1 303.495.2073
© 2025 Medical Affairs Professional Society (MAPS). All Rights Reserved Worldwide.
The healthcare ecosystem is becoming increasingly competitive and unpredictable and the volume of medical publications is doubling every 70-75 days, so companies in the pharma and biotech industry have recently begun to share data and collaborate publicly across more channels of communication than ever before. They are implementing new analytics technologies to increase the speed and quality of drug development and accelerate patient access to the best treatments or clinical trials. Recognizing that innovation takes competition, collaboration as well as risk and courage to share information in a data-driven environment, Amedea Pharma organized the first-ever Medical Affairs Innovation Olympics in life sciences with the slogan “Dare to Share for Our Healthcare”.
Competitors from companies such as Takeda, Astellas, and Roche among others submitted their ideas transparently, and 19 expert judges from 12 different companies as well as attendees evaluated the concepts across multiple rounds, semi-finals, and finals over one month. The event changed how we communicated innovative ideas across different companies transparently, how we debated, provided feedback continuously and in real-time with live voting and remarkable shifts in leadership rankings. The Olympics also showcased a data science approach to performance reviews, created a new Learning & Development (L&D) community with shared cutting-edge resources, and continuous enhancement of ideas as authors retained full ownership of their ideas’ intellectual property. In fact, the event led to new business partnerships that were publicly disclosed and created new opportunities for multiple startups.
For Private On-Demand Access to the Olympics event with bonus presentation slides, concepts, case studies please visit:
Thus, many Medical Affairs professionals across the globe became first-time Innovation Olympians, responded to a bold challenge, celebrated competition, accelerated innovation together, and shared a memorable experience together.
This article highlights the winners and finalists across the event categories of Field Medical Affairs, Evidence Generation, Operations, and Scientific Communication who presented critical new solutions for Medical Affairs to transform conventional norms of communication to engage with healthcare physicians, generate and exchange actionable insights at scientific conferences, actively contribute to improving the quality of clinical decision-making, connect more patients to appropriate clinical trials, and create a diverse, global community of patients and caregivers in a global pharma organization to improve quality of care, disease state expertise and quality of clinical trials.
Pharma access to healthcare providers has been steadily decreasing by nearly 50% in the last decade. This constraint was further amplified by the pandemic and required Medical Affairs divisions to rapidly adapt and also created a need for new engagement tactics. Additionally, physician satisfaction with MA digital engagement has been significantly low with only 25% of HCPs stating that they are satisfied with the current state. This is especially problematic because over the two years, over 75% of HCPs were driven to seek and consume clinical data on innovative medications, in order to update their current treatment strategies. This was especially the case with regard to their complex patients in the setting of COVID-19. As a result of this current unmet need, it has prompted the industry to actively pursue omnichannel engagement strategies to deliver relevant clinical data, drive valuable scientific exchange and create better digital experiences for Healthcare Providers overall. However, although the concept of omnichannel engagement has been extensively discussed within the industry, there are very few solutions, if any, that currently exist to serve the needs of Medical Affairs specifically. With this in mind, Discreedly was created to help address this unmet need and serve as an integrated omnichannel solution for Medical Affairs. Market research has demonstrated that more than 75% of HCPs find the current digital engagement strategies by MA to be rather generic and nearly 60% of providers also feel that current virtual platforms are lacking in overall quality and value. Therefore, it is imperative that medical affairs divisions transform their current digital engagement approaches and increase their capabilities. Companies that clearly understand these unmet needs, also recognize the OPPORTUNITY that they now have to elevate their medical affairs teams, to drive the SCIENCE behind their products and quench these needs for HCPs. 
In a nutshell, Discreedly is a digital platform that will help scale the engagement between pharma medical affairs and practitioners, to improve patient outcomes! With Discreedly, MA can engage effectively with HCPs and streamline the most current and relevant data to specific types of providers, through various types of formats and based on their current needs. Through Discreedly, companies can also increase scientific exchanges between HCPs and Field Medical (MSL) / MI teams (and other MA functions) to help provide valuable education, build scientific relationships, and gather valuable insights. The various engagement capabilities, including the bespoke on-demand tools, will help serve the pharma industry in multiple facets. These tools will help pharma drive awareness of the medical affairs division and their different functions (i.e., MSLs.) to a variety of HCPs in various practice settings. More importantly, previously unreached, and underserved healthcare providers can be engaged by MA to provide equitable access to the data, education and resources that are readily available and improve patient outcomes in communities with healthcare disparities. The customer base for Medical Affairs is widening as the demand for awareness and access to clinical data on novel medications continue to rise. The onus is now on Medical Affairs to deploy a needs-based engagement model and be an important partner for HCPs in their delivery of value-based care. Discreedly is developing a number of different tools and capabilities for medical affairs in the platform.
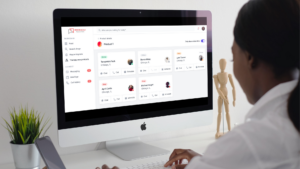 A key feature to point out is the dynamic analytics dashboard that medical affairs can utilize in a variety of ways including assessing HCP data needs; evaluating activities toward a product strategy; driving value narratives within the company; and demonstrating impact on patient centricity, just to name a few. As the digital world continues to evolve, today’s healthcare providers are very digitally astute and will only continue to increase as the next generation of HCPs enter the landscape. The analog methods and engagement model of medical affairs is fundamentally evolving. The pandemic expedited this change in tide and Discreedly aims to be a key partner for this transformational shift!
A key feature to point out is the dynamic analytics dashboard that medical affairs can utilize in a variety of ways including assessing HCP data needs; evaluating activities toward a product strategy; driving value narratives within the company; and demonstrating impact on patient centricity, just to name a few. As the digital world continues to evolve, today’s healthcare providers are very digitally astute and will only continue to increase as the next generation of HCPs enter the landscape. The analog methods and engagement model of medical affairs is fundamentally evolving. The pandemic expedited this change in tide and Discreedly aims to be a key partner for this transformational shift!
(If you are interested in conducting a pilot and would like to learn more, please email us at [email protected] or visit us at www.discreedly.com)
BRINGING MEDICAL CONFERENCE COVERAGE INTO THE 21ST CENTURY WITH inVISION
While medical conferences are key sources of information for your Medical Affairs teams, planning for the conference, managing schedules of multiple team members, gathering the important information from the massive amount of data available, and developing insights can be overwhelming. Another key challenge Medical Affairs teams have is longitudinally linking information from conferences over time so their teams can easily access historical content as well as gain insights from analyzing trends over time. inVision was designed specifically to help biopharmaceutical teams optimize conference attendance.

If you are interested in learning more about the best-in-class, cloud-based conference management system, visit www.inphronesis.com.
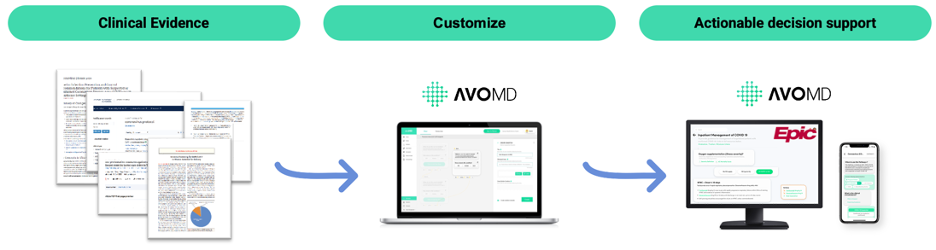
AvoMD is a platform for clinical decision support. The company partners with medical societies for authoritative clinical guidelines, creating nationally applicable, society-endorsed, standard-of-care algos based on the clinical guidelines.
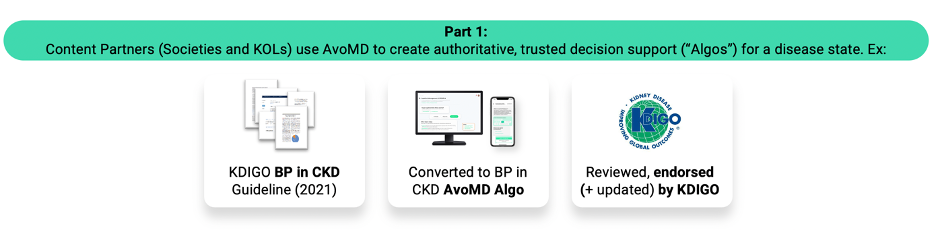
These algos are available “as is” to clinicians, or it may be customized to local-based pathways and practices using AvoMD’s no-code builder. The builder requires no programming knowledge, and thus saves health systems the need for IT or informatics. For clinicians, these algos save >50% of their time at the point of care, while increasing diagnostic accuracy, as they can pull in labs from patient context, and generate suggested orders and documentation.
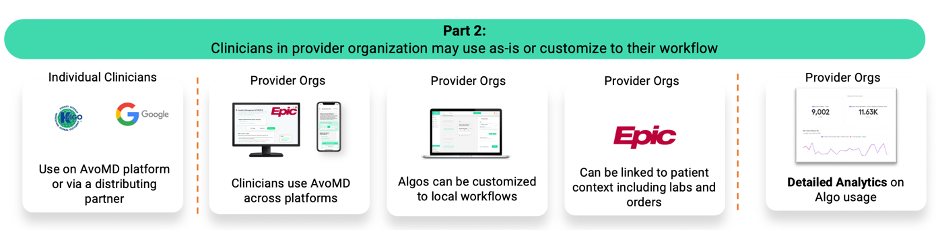
Life sciences companies have longed for decision support tools that promote the standard of care, when their products are already in the guidelines as standard of care. For life sciences companies, we provide a “last mile solution” that gets their products that are already in the guidelines into clinicians’ hands in a way that is compliant and ethical — as the content is already produced and endorsed by authoritative medical societies. We provide analytics, a distribution tool, and help market with the life sciences companies.
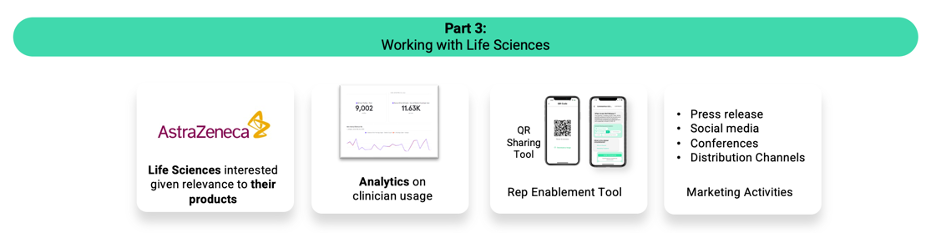
AvoMD transforms guidelines into actionable decision support at the point of care via EHR integration or standalone. By partnering with medical societies for content, allowing healthcare organizations to customize the content, and providing clinicians with actionable decision support algos, AvoMD is a major nexus in healthcare delivery. For life sciences, AvoMD is the last-mile solutionto bring products from the guidelines to the bedside.
For more information, visit: https://www.avomd.io/about-us
CareRing – connecting our lives with our science, Roche’s internal community for employees that are patients and caregivers.
A patient can be a Roche employee and any Roche employee can be a patient.
“CareRing has allowed me to connect with others that have similar experiences across the globe as well as allowing me to learn about initiatives from different parts of the business where I can add value by sharing perspectives from my patient journey.” – Nasam, a patient and member of CareRing
CareRing is Roche’s safe place for our colleagues who are also patients and caregivers –
regardless of the medical condition(s) or disabilities. It is a social media based community
allowing members to connect with others who are in a similar situation, to share their personal
stories and bring mutual support (whilst remaining anonymous through an avatar name).
Someone living with a medical condition often becomes an involuntary expert and therefore
understands best what needs to be done to improve daily life. CareRing offers many (voluntary)
opportunities to get involved in disease areas that are personally relevant through projects and
co-creation activities. Through these interactions, Medical Affairs and other business functions
are better able to understand the unmet needs of people directly affected by a condition and
can incorporate these learnings into the design of their research and development strategy,
thereby ensuring the best possible outcome for patients and their family. Employees who
become patients can now influence what Roche is doing and make sure the patient’s voice is
heard and listened to.
Collaborating with CareRing members allows our business/project teams to bring in the
patients’ and caregivers’ experience earlier in our strategy and planning so we can develop
hypotheses and prototypes that can be validated through the usual approaches. Collaboration
with CareRing does not replace engagements with external patients associations or market
research; it complements it by allowing us to co-create together with patients and caregivers
from the very beginning of a project.
CareRing was founded in January 2020, it has a dedicated community lead and a steering
committee. The community counts more than 1000 members in 60+ countries and 170 projects
were submitted, e.g. building on insights shared by the AD CareRing sounding board, it enabled
the creation of a comprehensive guidebook that supports clinical trial design and management
to encompass the needs of participants and their study partners. The external report now
brings the learnings from this guidebook to a wider audience (researchers, industry members,
patient advocacy groups, and the Alzheimer’s disease community).
CareRing is a key partner to drive the patient centered transformation in Roche and through
outreach to other pharma companies, a better understanding on how others can implement it
and benefit in a similar way.
For more information, visit: https://carering-community.roche.com/

Money can’t buy freedom. Nor can it directly cure a disease itself.
If you have a close relative with a chronic disease, you might have experienced the feeling of being powerless and frustrated due to lack of options to help. Maybe you have even a chronic disease yourself. Clinical trials is the most time-consuming part of developing new therapies. Chronic patients and researchers know exactly what this is about. Trial participation and recruitment are two sides of the same problem.
Chronic patients ranging from patients with type 2 diabetes, to ALS, need to navigate through a jungle of irrelevant trials to find the one they might get enrolled in. Patients usually have no chance of knowing whether they suit a trial or not. And when they finally do find a trial, they’re bombarded by screening-questions or best-case sent a questionnaire/form to will with data they don’t know.
Researchers have limited time and heavy regulatory restrictions to recruit trial participants. Reaching out and screening patients for trials is by far one of the key drivers in inefficient recruitment and drug development. Furthermore, it’s difficult to understand the balance between the quality of the data vs. how demographically realistic the criteria are.
It is estimated that 30% of trial participants drop-out after enrollment, and much data indicates that this mainly is due to the poor initial understanding of the participant-information. Recruiting a patient is estimated to cost avg. 6,533USD, while replacing a patient is even higher costing around 19,533 USD on avg.
So how do we make clinical trials an option for hope, and not a burden? How do we make sure that fast and high-quality evidence is generated? How can we make treatments reach the patients faster?
PROBE is a revolutionary new platform for clinical trial recruitment/participation. Our web and mobile-based platform empower individuals to take control of their own health and contribute directly to important medical research. With the help of health data and a integrated rigorous matching algorithm, users can get suggestions on recommended trials, based on their health data.
With strategic data-entry-points, we help boarding and updating the data, and uploading it to one of the world’s most secure cloud-databases. A user-friendly and patient co-created overview of the trial is then presented, and the user can simply hit “Apply”. No screening process, fewer misconceptions, and fewer disappointments on getting rejected to trials.
Join us in our mission to revolutionize clinical trial recruitment, making it accessible and make a real impact on the future of medical research. Contact [email protected] to hear more and get updated in the journey of the development of PROBE.
For more information: www.copenhagenhealthinnovation.dk/health-innovators-presents-the-winning-start-up-probe/

 2023, the year of personalization? Omnichannel principles for Training Stra...
2023, the year of personalization? Omnichannel principles for Training Stra...