Collecting, Curating, and Activating Medical Affairs Knowledge in the AI Era
By Stephen Casey & Jenniffer Shannon, M.D.
“Knowledge is everything” and organizations utilize knowledge to drive success, but collecting, curating, and activating knowledge is one of the most difficult challenges for life science companies.
A high-quality medical knowledge base is the foundation to ensure accuracy, safety, and compliance when using generative AI tools.
In this three-part series, we discuss the evolution of the medical affairs knowledge base and how the rapidly changing landscape of generative AI is shaping this. We will discuss the process of knowledge management and the critical role that a high-quality, validated, and centralized Medical Affairs knowledge base plays in optimizing departmental and organizational functions.
Part I – Medical Affairs Knowledge Base (MAKB) Creation
Article Takeaways:
- Effective scientific knowledge management is not merely an operational necessity; it forms the bedrock upon which life sciences organizations operate. It is critical for medical affairs to facilitate the discovery, application, and timely knowledge transfer within the organization
- Collected data is currently fragmented, trapped, and scattered in different files, formats, and behind access walls within an organization. Knowledge management is a time-intensive and manual process becoming increasingly difficult due to the ubiquity of information and exponential growth in scientific data.
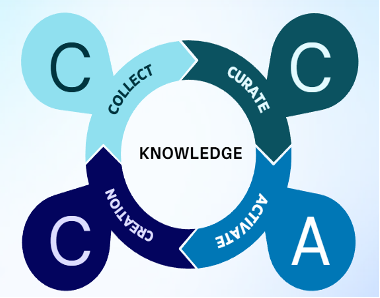
- We overlay the Collect, Curate, Activate Framework to enable Medical Affairs teams to effectively and accurately leverage medical knowledge and AI tools
The Evolving Role of Medical Affairs (MA)
MA has evolved over the years from being a reactive support function to a forward-looking, strategic partner especially in scientific exchange and evidence generation.
However, with that shift, there have come increasing responsibilities to juggle. Today’s medical and scientific teams face overwhelming amounts of data ranging from conferences and clinical trials to peer-reviewed literature, advisory boards, and KOL insights. Traditional methods no longer suffice to keep up with the speed and complexity of the data.
The Importance of the MAKB
The MAKB is a centralized source of ground truth and scientific rigor for the company to build upon. It plays a critical role as the evidence base that drives departmental and other cross-functional initiatives in the company.
COLLECT
MA teams currently collect and transform data into knowledge and insights through a complex process, where experts review, validate, and rank scientific information to ensure accuracy and compliance. This data is collected from multiple data streams such as KOL meetings, advisory boards, conferences, literature, and other areas.
CURATE
Selecting and ensuring the accuracy and consistency of data is critical, yet scattered repositories continue to make data access difficult and organization challenging. The meticulous curation of scientific data involves a systematic process of selecting, organizing, cleaning, and maintaining data to ensure it remains accessible, reliable, and valuable. This includes annotating, cataloging, and enhancing data. The goal of data curation is to manage the knowledge into a MAKB so it can be easily found, accessed, and retrieved.
ACTIVATE
For centuries, knowledge was handed down through personal interaction or physical storage areas. Individuals within the organization would collect and curate knowledge. However, activation of that knowledge was problematic. Access and retrieval of the knowledge was challenging due to location and structure to the data usually resided with one person. This was overcome to some extent with the computerization of the office and the introduction of relational databases. But even relational databases, which could not provide context and understanding, limited the ability to derive insights.
Today, most data and insights remain trapped, disconnected, and scattered in static data bins everywhere: employees’ brains, CRMs, SRLs, SharePoint folders, PowerPoint slides, etc. Like using a filing cabinet, retrieval of this knowledge requires manual and often inefficient processes making it difficult to activate the data to be used again.
A well-constructed and validated MAKB fosters rapid responsiveness and crucial knowledge sharing across diverse teams. In today’s environment, MAKBs are critically important to generate the insights and information we seek. Moreover, the MAKB is central to ensuring compliance with stringent regulatory standards and adherence to ethical conduct, safeguarding the integrity of the organization and the well-being of patients.
Building a high-quality MAKB requires a strategic understanding of the collection, curation, and activation processes to deliver optimal outcomes without redundancy. Properly curated (analyzed, classified, referenced, and linked) MAKBs lead to data readiness today and into the AI future.
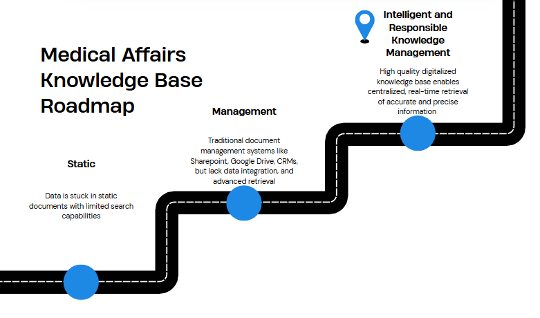
The Current MAKB
- Current systems fall short: MA teams continue to face challenges in quickly accessing relevant information despite increased usage of CRMs, document management platforms and enterprise copilots. These difficulties stem from outdated search and filtering capabilities, low-quality or incomplete databases, and the absence of grounded datasets to support intelligent retrieval.
- Missed insights and data silos: Critical relationships and insights are missed due to the lack of a structured MAKB supported by efficient discovery across vast and disparate scientific and enterprise datasets.
- Increasing compliance demands and time sensitive tasks: It is increasingly difficult for teams to keep pace with rapid scientific updates, sift through overwhelming amounts of information, respond quickly to medical inquiries and keep up with compliance demands.
Companies are recognizing the major drawbacks of their MAKB and are trying to improve their processes of medical knowledge collection, curation, and activation.
Using standardization and document management systems like SharePoint and Google Drive, MA professionals have been able to make access and retrieval easier. Some have even progressed beyond simple sharing and have started integrating with CRM systems like Veeva or Salesforce to enable better management, but significant challenges and limitations remain.
Although these systems provide some level of search functionality, often relying on basic keyword matching and filters, MA teams still lose considerable time and resources attempting to locate the precise information needed at the right moment.
A large part of this is because the data is not stored or structured in a way that enables deeper questions which require multi-step reasoning or interconnection of multiple data points or because the data does not embed temporal information and therefore is not real-time or up to date.
This struggle can lead to missed insights, as relevant connections between disparate pieces of knowledge are not readily apparent. Furthermore, the lack of a robust infrastructure to organize the growing volume and complexity of scientific data contributes to ongoing data silos, hindering a comprehensive understanding and resulting in decision making based only on the information that is easily retrieved. The continued reliance on manual processes remains a bottleneck, slowing down access to critical information.
Adding to these operational challenges are the ever-increasing demands of rapidly changing compliance requirements in a high-stakes’ environment.
MA professionals face constant pressure to generate accurate medical information and content rapidly, particularly in response to time sensitive situations like medical inquiries. The swiftly expanding and increasingly intricate landscape of scientific data, including RWE and new publications, necessitates the ability to find information quickly and ensure real-time updates. All of this has to be navigated without running afoul of increasingly complex data and privacy regulations which add another layer of difficulty.
The Onset of Generative AI: Risks, Benefits, and Opportunity
It is natural that MA teams turn to AI tools to improve efficiency and productivity. However, without first establishing a high-quality, vetted knowledge foundation and the right data infrastructure, organizations risk defaulting to generic large language models (LLMs) such as ChatGPT or basic internal copilots which introduce significant compliance and safety risk. These generic LLMs are not rigorously evaluated and grounded in organization-specific medical knowledge, which often results in poor-quality and unreliable outputs, e.g. hallucinations and inaccuracies, introducing significant compliance and safety risk.
The Future of MA Knowledge
- A high-quality MAKB is essential to enhance the strategic value of MA by enabling integration, connection, and analysis of multiple data streams and insights
- Many life science companies are planning or are already incorporating Gen AI, but without starting with a high-quality knowledge base, data governance, evaluation, and safety testing, deploying Gen AI applications is risky
The ability to definitively measure the value contributed by MA is becoming increasingly important and as the keeper of scientific knowledge, a sophisticated MAKB will be instrumental in amplifying MA’s impact in the future.
The continuous development and updating of the MAKB is also critical to ensure its relevance and utility in a rapidly evolving scientific landscape. A key driver of this evolution is the growing significance of artificial intelligence and its diverse applications in life sciences organizations. A robust and well-structured MAKB will be critical for grounding AI models, validating their outputs, and ultimately augmenting human expertise across the organization.
Over our next two articles in this series we will discuss the optimal MAKB, how it will facilitate the use of trustworthy AI, generate higher quality insights and deliver the right information in the right format to diverse stakeholders. Look for our next installment in the coming weeks or follow us on LinkedIn to be notified.