BUILDING MEDICAL INSIGHTS CAPABILITIES IN MEDICAL AFFAIRS ORGANIZATIONS
Authors
Matthew McLoughlin, CEO, Synetic Life Sciences
Marieke Jonkman, Medical Science Liaison, QED therapeutics
Milana Zivkov, Senior Director, Insights and Training Global Lead, Medical Communications, Medical Affairs, Astellas
This publication represents the consensus opinion of members of the MAPS Insights Focus Area Working Group (FAWG) but does not represent formal endorsement by the authors’ organizations.
Introduction
Pharmaceutical companies continue to evolve the way they operate, both internally and within the external environment. A variety of factors drive this evolution including societal expectations, continued changes in healthcare systems across geographies, advances in digital technology and its application (as highlighted during COVID 19), and complex legal, regulatory, compliance, and privacy obligations. As a result of these factors, the Medical Affairs (MA) function will become increasingly critical due to its unique positioning, as follows:
- Externally, MA engages with stakeholders in a non-promotional/ scientific manner to understand the patient journey from the perspective of all key stakeholders including patients, HCPs, and payers
- Internally, MA interacts with cross-functional and cross-divisional stakeholders throughout the value chain
- MA can also act as a mediator between the internal and external environments, driving strategy and innovation through scientific and patient-centred medical insights.
This paper explores the MA-based medical insights function and insights-related activities from an MA operational perspective, and identifies basic operational elements required to establish medical insights as a core strategic MA function.
“Medical Affairs stands to be one of the most strategically important and valued functions in a pharmaceutical company, as the nexus of cutting-edge medical, scientific and patient-centred insights that drive strategy and innovation throughout the entire product life cycle”
—McLoughlin and Croft (2018), Medical Affairs 2025, The future of Medical Affairs, Kinapse 2018
Medical insights are a cornerstone of the Medical Affairs value proposition
Medical insights from the external environment have always been important to the pharmaceutical industry, but the nature and sources of insights have evolved. HCP opinion was traditionally gathered in a targeted way through advisory boards and individual consultations. While this approach continues to generate key medical insights, there is now recognition of the opportunity to develop earlier and more comprehensive understanding of external stakeholder needs (particularly patients and caregivers). This requires MA organizations to expand their approach, combining the following:
- Multiple channels to gather information from the external environment e.g., patient ad boards, social media listening, publications etc.
- Technology to collate, curate, analyse and interpret information gathered
- Operational structure to share and utilise that information, identify medical insights and manage the overall insights process
“An insight is new information, understanding, ideas or perspectives on topics relevant to a company that may identify a gap, and/or inform strategy, and/or confirm pre-existing views held by the company and may result in an action where and when appropriate.”
— MAPS Insights Glossary
The Insights FAWG Medical Insights surveys
Given the increasing focus on medical insights, the MAPS FAWG conducted two surveys of MAPS membership in early 2020. The surveys highlighted the following key points:
- There was wide variation in how companies approach medical insights, with no apparent areas of best practice or common frameworks in use.
- Approximately half of the respondents have an MA function defined as responsible for medical insights; however, standalone Medical Insights functions are relatively infrequent and exist in less than a third of respondents’ organizations.
- For many companies, Field Medical-driven activities appear to be the focus of insight generation, with limited use of some other channels (e.g., only 26% of respondents systematically collect insights from scientific literature, and only a quarter collect insights from social media sources).
- There appear to be challenges in creating a closer link and feedback loop between those working at the customer interface and those setting strategy, including identification of ‘real insights’ and linking them to action.
- There appears to be consensus across the industry on the relevance of medical insights as a principal driver for strategy determination and choice of tactics. However, the survey results suggest that the everyday practice of medical insight generation is fragmented – in 47% of respondents’ organizations (Survey 2), insights generated across different sources are not integrated.
Although there is often consensus on the importance of medical insights, the remit of an MA function in medical insights varies. Some organizations regard MA insights as an add-on to competitive intelligence and market research exercises led by Commercial. Where more mature capabilities are available, companies seek MA input on cross-functional compound/product strategy development from credible and comprehensive medical insights based on patient, HCP and payer needs and value drivers.
What can MA organizations do to optimize medical insights-related activities?
As confirmed by the MAPS Insights FAWG surveys, there is wide variation across the industry in how companies approach the generation, curation, reporting, sharing and utilization of MA insights. Too often, digital technology focused on the Field Medical function is the first reference point when companies attempt to develop in this area. In this paper, we propose a more holistic approach to the MA operating model for driving medical insights activities, recognizing interdependencies between the components.
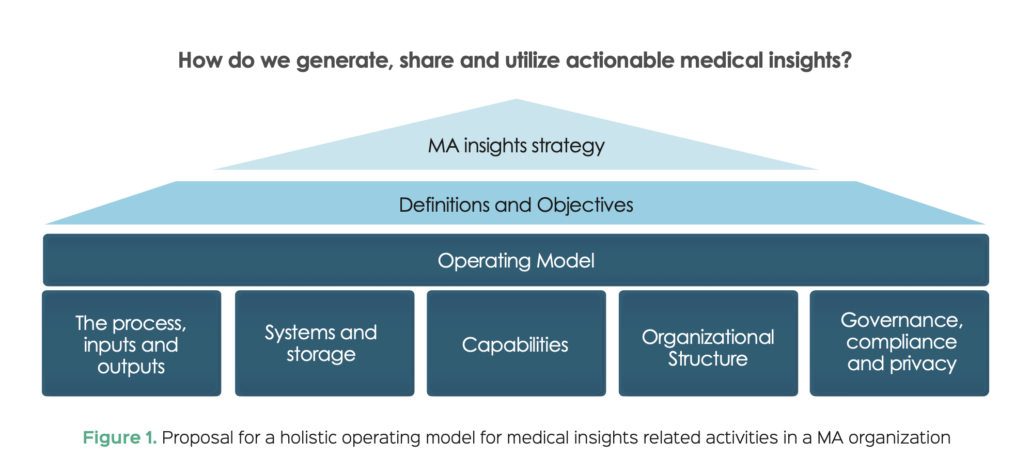
Figure 1. proposes a holistic framework through which to consider design of an MA operating model for insight-related activities, with foundational elements we believe are critical to success. Each of the foundational elements will subsequently be discussed in more detail.
Definitions and objectives aligned with MA insights strategy
As with the design of any operating model, the first challenge is to align on and communicate what we are trying to achieve. While the aspiration may be to have Medical Affairs lead the integration and analysis of ‘Patient’ insights from all channels, this can be complicated in large companies. We have identified the following three key factors at this stage:
- A clear definition of medical insights that delineates the remit of the MA organization from competitive intelligence or commercial insights and helps to clarify the role of medical insights in better understanding of the needs of patients and other stakeholders in the healthcare ecosystem
- Internal clarity on medical insights as a core MA activity performed to drive strategic and tactical choices which helps to determine the approach to building medical insights infrastructure
- Understanding of how insights are regularly reviewed and utilized to adapt tactics throughout the year, as opposed to generating insights and reports without a clear idea of how and when to use them
For the purpose of this paper, we will focus on medical insights as defined in the MAPS Insights Glossary, i.e., as new information, understanding, ideas or perspectives received from external sources through MA-led activities, and pertaining to medical topics relevant to the company, including but not limited to the general healthcare environment, diagnosis and unmet medical needs, treatment patterns, patient journey and specific regional or local issues.
The process, inputs, and outputs
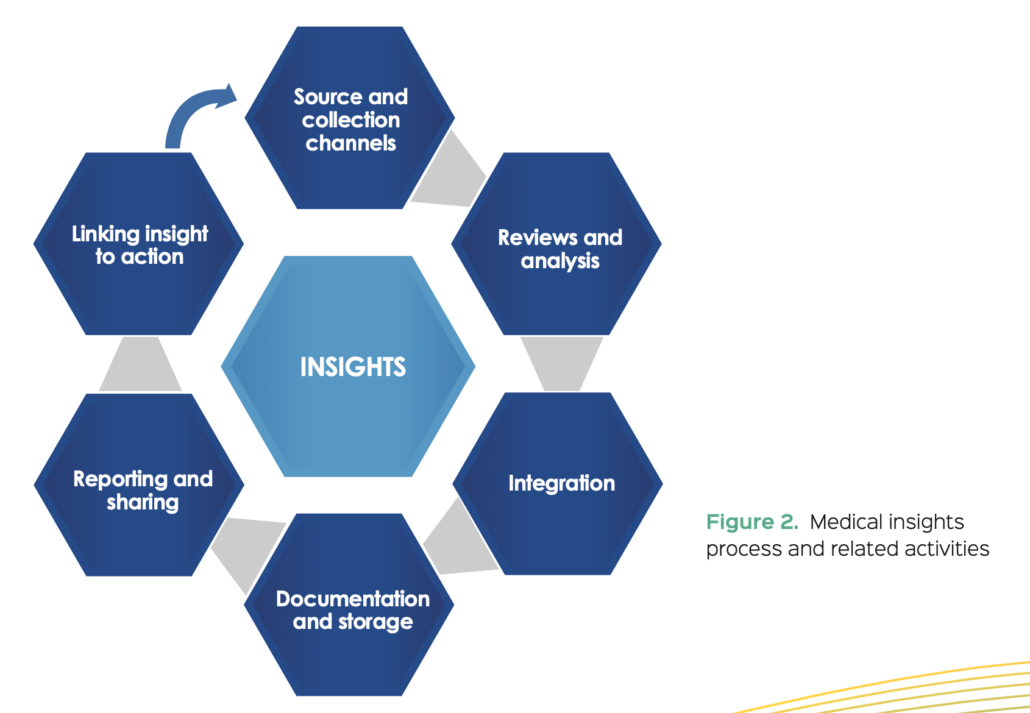
Defining and documenting the process
The core elements of the medical insights process are shown in Figure 2. The process will naturally vary in complexity depending on factors such as company size, disease area breadth, product life cycle stage, portfolio, and integration of geographies and functions into the MA organization. Ultimately, process design will depend on how insights are used. Medical insights could be reviewed and combined with insights from epidemiology, safety, clinical and commercial at a country or regional level, or this could happen at a global brand or therapy area level, depending on the needs and structure of the organization. The optimal approach for a given company will depend on how the insights inform action and strategy and will also reflect organizational structure. Irrespective of the factors described above, the steps in Figure 2 serve as a basis for establishing a medical insights process in any MA organization.
Sources of insight and inputs to the process
The surveys highlighted that many MA organizations consider insights to be limited to those that Field Medical/MSLs gather from external scientific and clinical expert interactions. This perspective unnecessarily limits the scope and value of what is possible for an MA organization. While some sources of information and insights are more accessible than others, organizations should consider all external interactions (through digital portals, virtual or in-person meetings) across all MA functions as potential insight gathering opportunities. This includes, but is not limited to, interactions between MA and HEOR representatives and external stakeholders, Medical Information, Advisory Boards, consultations, or Medical Education. In addition, technology that enables automated searching and analysis of structured and unstructured, quantitative, and qualitative, data is much more widely available and tailored to the life sciences industry than it was several years ago. Technological evolution now enables social media listening, as well as similar analyses of external publications, internal data sources, Adverse Event reports and other data bases containing unstructured qualitative data.
Given the vast array of data sources available, the framework defining which sources should be routinely analysed within an MA organization becomes key. Ultimately, this framework should be organised by strategic pillars or themes that are relevant to the users of insights, rather than by insight source. A fit- for-purpose operational framework will aid the integration of data and information, particularly in large and complex organizations that use a multi-channel approach to insight generation.
Outputs and recipients of insights
Drawing on feedback from the survey, one of the most important aspects is to define what purpose medical insights serve and for whom. This enables the MA organization to create a feedback loop between those working at the external stakeholder interface and those setting strategy, helping to generate actionable insights and ensure streamlined insights-related activities.
Determining which stakeholders are primary insights recipients versus secondary recipients is essential to determining the nature of the output, its format and frequency, and any associated commentary. Figure 3 outlines considerations for optimizing medical insights reports based on feedback from insights recipients. Primary internal recipients will vary between organizations, but in most cases will include MA, HEOR, Product, Region and/or Affiliate leaders, as well as therapy area leads. Other MA leaders, such as external stakeholder engagement, field medical, scientific communications or knowledge training leads, are also becoming primary internal stakeholders due to MA organizational diversification and specialisation. In contrast, leaders outside of MA may be primary or secondary recipients.
Systems and storage
Almost half (47%) of survey respondents reported the use of CRM systems to store medical insights at their organizations. However, CRM systems tend to be oriented towards the external stakeholders with which an MA organization interacts. This is useful for field medical colleagues in terms of insight collection and use of insights to inform future interactions. However, a more fit-for-purpose data repository or platform is required if insights are to be used as a resource to drive strategy and action in broader internal stakeholder groups. Such a platform must support the stages of the medical insights process in Figure 2., and should ideally enable the following:
- Upload of information from different medical insights-generating functions and sources, from both validated internal systems (e.g., CRMs and MI systems) that collect structured information and data, as well from other internal tools used to collect and store non-structured information and data (e.g., narratives or slide decks stored on SharePoint or other internal repositories)
- Automated curation of insights, and their categorisation around dimensions that are relevant to the MA organization and medical insights users
- Automated creation and delivery of reports and metrics relevant to the organization, linking to the already established metrics, analytics or reporting tools used in the organization (e.g., Tableau)
Availability of a user-friendly interface that facilitates information or pre-insights submission and provides guidance on insights the MA organization is looking for can also be helpful.
Capabilities
Capabilities should be considered both in terms of organizational capability as well as individual competencies. The former is improved as processes and systems evolve, and as internal stakeholders become more familiar with what works and what does not work, as well as where companies should focus resources and effort to maximise impact.
The organization must consider individual competencies more deliberately and proactively. To achieve insights excellence, medical insights-focused MA colleagues need to be appropriately and systematically trained to be:
- Familiar with the medical insight generation process and function-related process specifics
- Proficient in narrative writing, where applicable
- Aware of information/data that may lead to insights
- Proactive in identifying and raising pre-insights
- Capable of applying both analytic and synthetic methods to identify any major data or temporal trends in generated insights
- Able to work effectively with niche external providers of data and/or technology
- Familiar with the reporting, utilization, and feedback process
One of the options for such an approach is to integrate insights-focused training as part of the onboarding process for new hires in MA, and to have annual refresher courses to drive best practice sharing between contributing geographies or functions.
Organizational Structure
There has been much debate on whether a Medical Affairs organization benefits from, or even requires, a dedicated ‘insights group’. The two key issues are to distinguish the set-up of an insight capability from the ongoing operations, and to be clear about the scope of such a potential group.
The medical insight capability often sits within Medical Operations or Medical Excellence. The location of an insights capability within Medical Operations is often driven by various factors including broader organizational structure, individual competencies and interests, and any groups which have successfully pioneered processes or technology. Alternative locations include within Medical Information, within a digital group, or in a capabilities or planning group.
Regardless of where it sits, a central medical insights group drives the development of capability through learnings and best practice. Importantly, it also provides one point for the application of AI and automation technologies as well as the associated outsourcing and data access options.
The more substantial issue is the scope of responsibilities of such a group moving forward. A dedicated group could be tasked with collating and curating information and producing insights reports for the organization. Alternatively, they could be tasked with creating and maintaining a compliant framework to enable therapy areas, business units, regions, or countries to generate and codify their insights to be accessed and used across the business. This latter remit is preferable as it acknowledges the importance of involving individuals with deep strategic, therapeutic, and disease area understanding early in order to distil useful insights. As with Compliance or Patient Centricity, it also emphasises that gathering insights is a responsibility of every role in MA, albeit with the support of consistent definitions, templates, process guidance, data sources and information management technology.
Governance, Compliance and Privacy
Governance and Compliance are essential considerations when developing an insights capability, and the key topics for which a clear understanding from all stakeholders is required are:
- Reporting of adverse events, specifically where social media listening is used
- Adherence to data privacy laws and regulations when handling personal data
- Following appropriate processes when patients are engaged directly
These points then raise issues of how practices in particular countries, with unique laws and codes of practice, are managed through global company policies – inevitably this mirrors the approach taken to policies on external scientific engagement which are usually already in place.
The challenge that many companies face is not defining the ‘right answer’ to the issues mentioned above, but instead determining a position that is logical, consistent, and well understood. Consultation with Compliance and Privacy colleagues should occur prior to initiation of any MA medical insights activities (particularly at the global level), to ensure all rules and regulations are followed.
Summary and Conclusions
The results of two surveys of MAPS membership conducted by the Insights FAWG in 2020 suggest that there is consensus across the pharmaceutical industry on the relevance of medical insights as a principal driver for strategy determination and tactic selection. However, the results also suggest that the everyday practice of medical insight generation and utilisation is fragmented across MA organizations. To address the importance of medical insights and evident fragmentation of approaches to medical insights related activities across MA, organizations should seek to:
- Define clear definitions related to insights and develop objectives that are aligned with the overall MA insights strategy
- Develop a well-defined, documented process for the collection, review, analysis, integration, reporting and sharing of insights
- Leverage technology, especially digital, AI and advanced analytics, and new datasets to collate, analyse and share insights
- Define clear organizational roles and responsibilities, taking a strategic approach to partnering, licensing, and outsourcing
- Develop individual skills to optimise each step in the medical insights process, including the ability to work in complex digital environments
- Implement a fit-for-purpose and compliant data storage system with user friendly interfaces and reporting capabilities
- Ensure adherence to company policy, as well as local legislation (including GDPR and AE reporting)
This foundational framework should help MA organizations to streamline the medical insights process and related capabilities, and ultimately result in enhanced identification of high-quality, actionable insights to drive decisions on strategy and tactics.