INTRODUCTION
The Medical Affairs Professional Society (MAPS) is pleased to share the 2025 Medical Affairs Launch Readiness and Execution Benchmark Report. This report is based on findings from 28 leading organizations across Pharmaceutical, Biotech, and Medical and Diagnostic Device sectors, and reflects broad leadership thinking about the role of Medical in product launch, current and future challenges, and the capabilities required to succeed in the future.
Survey design and analysis:
Boston Consulting Group and MAPS led the design, execution, and analysis of the Launch Readiness and Execution survey and moderated the workshop during the 2025 MAPS Americas conference.
The survey covered five key areas:
- Current status of Medical’s role in launch
- Resourcing and capabilities
- Innovation and technology
- Measuring Medical Affairs’ impact and success in launches
- Gaps and opportunities
Survey respondents:
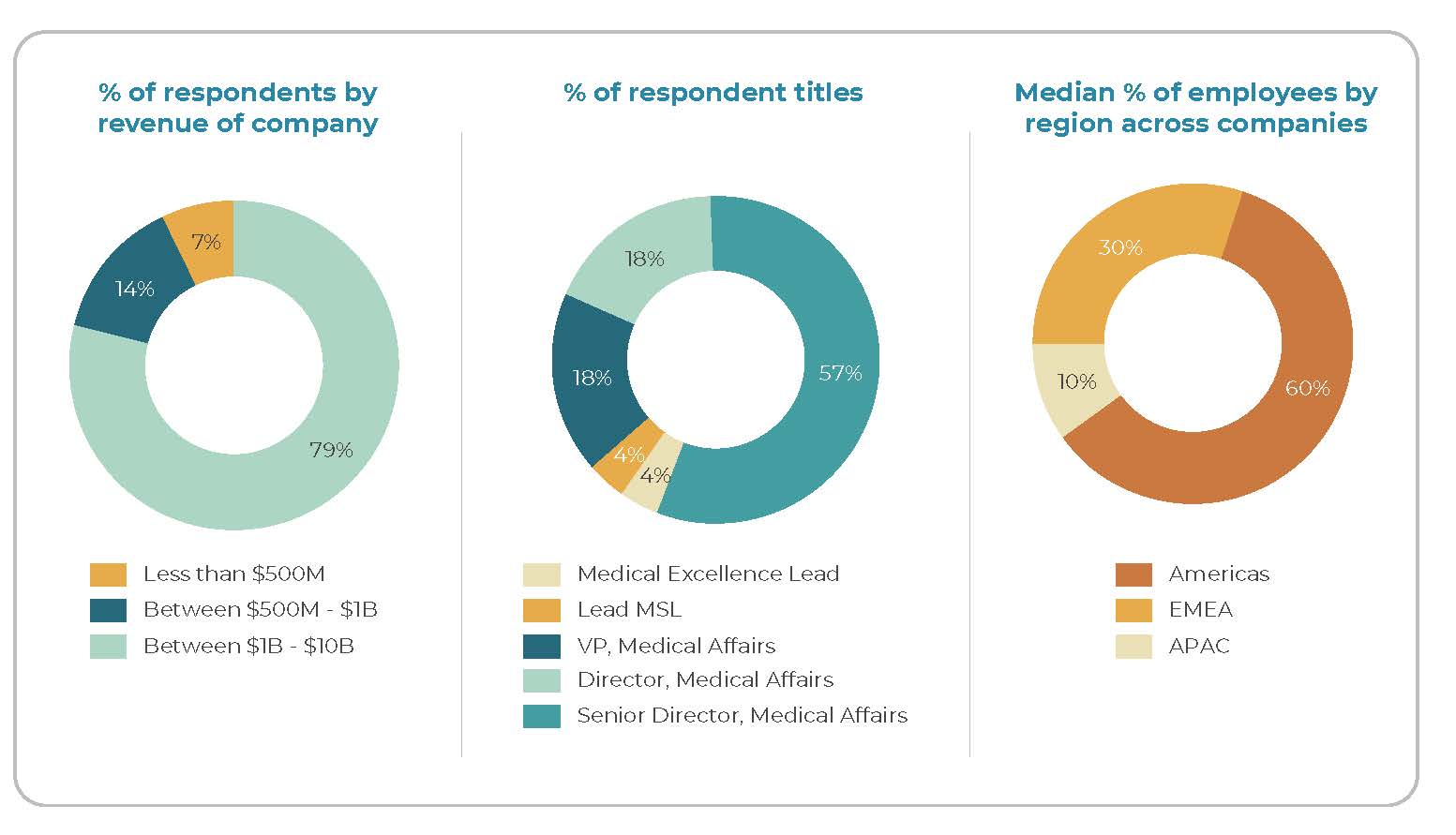
Responses were collected from 35 senior Medical Affairs professionals from 28 companies.
Respondents are Medical leaders, primarily at the director level and above, with responsibility for global or regional Medical functions.
Responses reflect both headquarters and field perspectives, spanning strategy, evidence, and engagement responsibilities.
Participating companies in the Survey:
- Abbott
- AbbVie
- Astellas
- AstraZeneca
- Baxter
- Bayer
- Biogen
- bioMérieux
- Bristol Myers Squibb
- Daiichi Sankyo
- Eisai
- Ipsen
- Jazz Pharmaceuticals
- Leica Biosystems
- Lundbeck
- Mallinckrodt
- Medtronic
- Novartis
- Otsuka
- Organon
- Pfizer
- Sage Therapeutics
- Sanofi
- Teva
- UCB
- Varian
- Vertex
- Xenon
Respondent demographics: 57% are Senior Directors, with Directors and Vice Presidents each at 18%. Companies’ footprints are global but US-leaning, with the median distribution of Medical employees at 60% in the Americas.