Generating better outcomes for patients through strategic planning
From the choice of where to publish to the assessment of impact, every decision holds weight in medical research publication. And yet, the metrics that have been traditionally used to guide these decisions offer only a partial view. The result: a hit-or-miss situation when it comes to reaching the target audience—healthcare professionals (HCPs) and patients. Can a data-driven approach that incorporates both traditional and the newer alternative metrics hold the key to improved publication planning, impact and thereby better patient outcomes?
Origins and limitations of traditional metrics
The idea of assessing a journal’s impact traces its roots to Eugene Garfield’s efforts in the early 1960s. Garfield introduced the concept of the Impact Factor (IF) to gauge a journal’s importance through citation counts. Despite its relevance at the time, IF faces inherent limitations. In 2012, the San Francisco Declaration on Research Assessment (DORA) questioned the sole reliance on IF for evaluating research quality. IF primarily reflects past performance, offering a retrospective view that fails to capture a journal’s current relevance. Furthermore, a journal’s IF is primarily driven from a handful of outliers with very high citation rates, and doesn’t even offer insights into other key metrics, such as whether an article has any citations whatsoever. The IF cannot predict the success of individual papers or account for the diverse ways in which research impacts the real world.
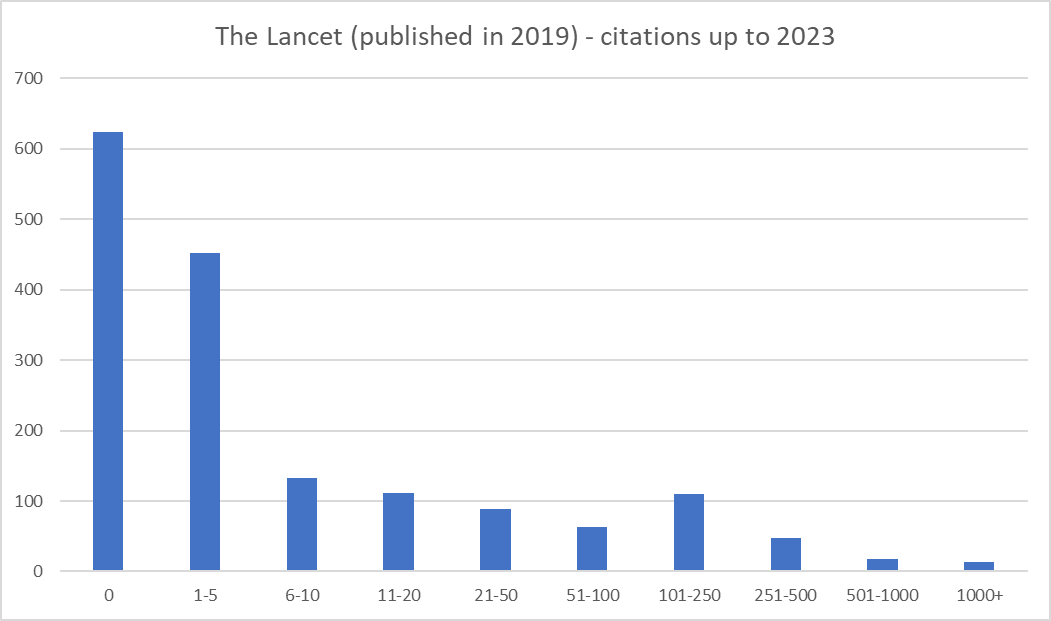
Figure 1: Number of (all) publications in 2023; Number of citations = 81,105; 31% still haven’t been cited; Another 29% have 10 or fewer; 2.5% contribute 50% of the citations; 79% of articles have Altmetric attention, mean is 114.
Evolution of metrics: Altmetric and derived measures
As computational power advanced, so too has our ability to quantify scholarly impact. Altmetric, which has been tracking mentions of research outputs across various online platforms for over a decade, is widely used by scientific publishers, researchers and academic institutions as a way to measure their broader impact. These forms of data offer a more comprehensive view of influence, capturing both scholarly and public engagement across a broad range of impact types, and demographics. In light of these advancements, publication planners must reassess the criteria for evaluating journals and reevaluate what constitutes a ‘good journal.’ Publication in a well-chosen journal can ensure that research findings are disseminated effectively to HCPs, leading to informed clinical decisions and ultimately better patient outcomes, independent of its standing in legacy metrics, such as the IF.
Selecting a good journal
So what factors, other than traditional indicators like Impact Factor and submission volume, are important when it comes to selecting a journal? Open Access status, editorial board relevance, and online visibility all play pivotal roles in determining a journal’s impact.
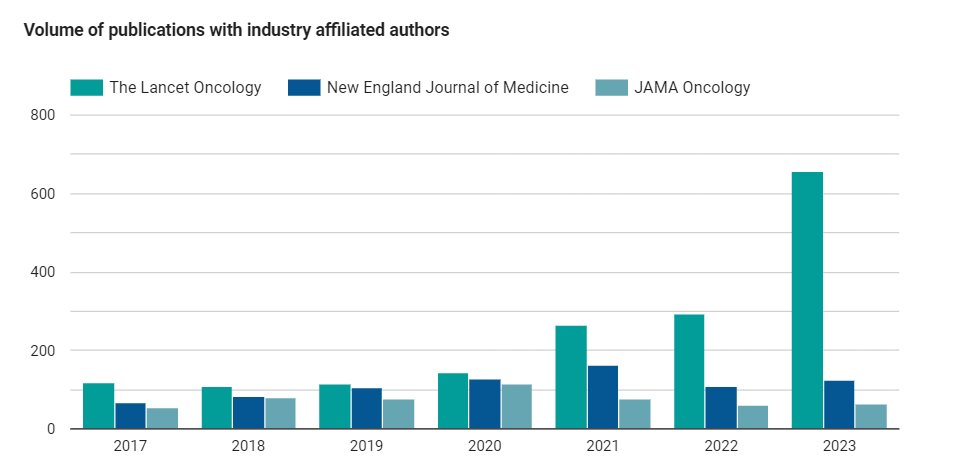
Figure 2: The volume of publications that have industry-affiliated authors in the Lancet Oncology, New England Journal of Medicine, and JAMA Oncology.
Data-driven tools like the Journal Selection dashboard enables publication planners to make informed choices about where to publish research findings. Such dashboards offer a holistic view of journal performance, allowing publication planners to make informed decisions aligned with organizational goals. By leveraging Altmetric and Dimensions data, this tool goes beyond traditional metrics, offering a quick means to access precise metrics and comprehensive understanding of a journal’s global impact. This helps users make informed decisions on where to publish their work, maximizing the reach and scientific impact of their research.
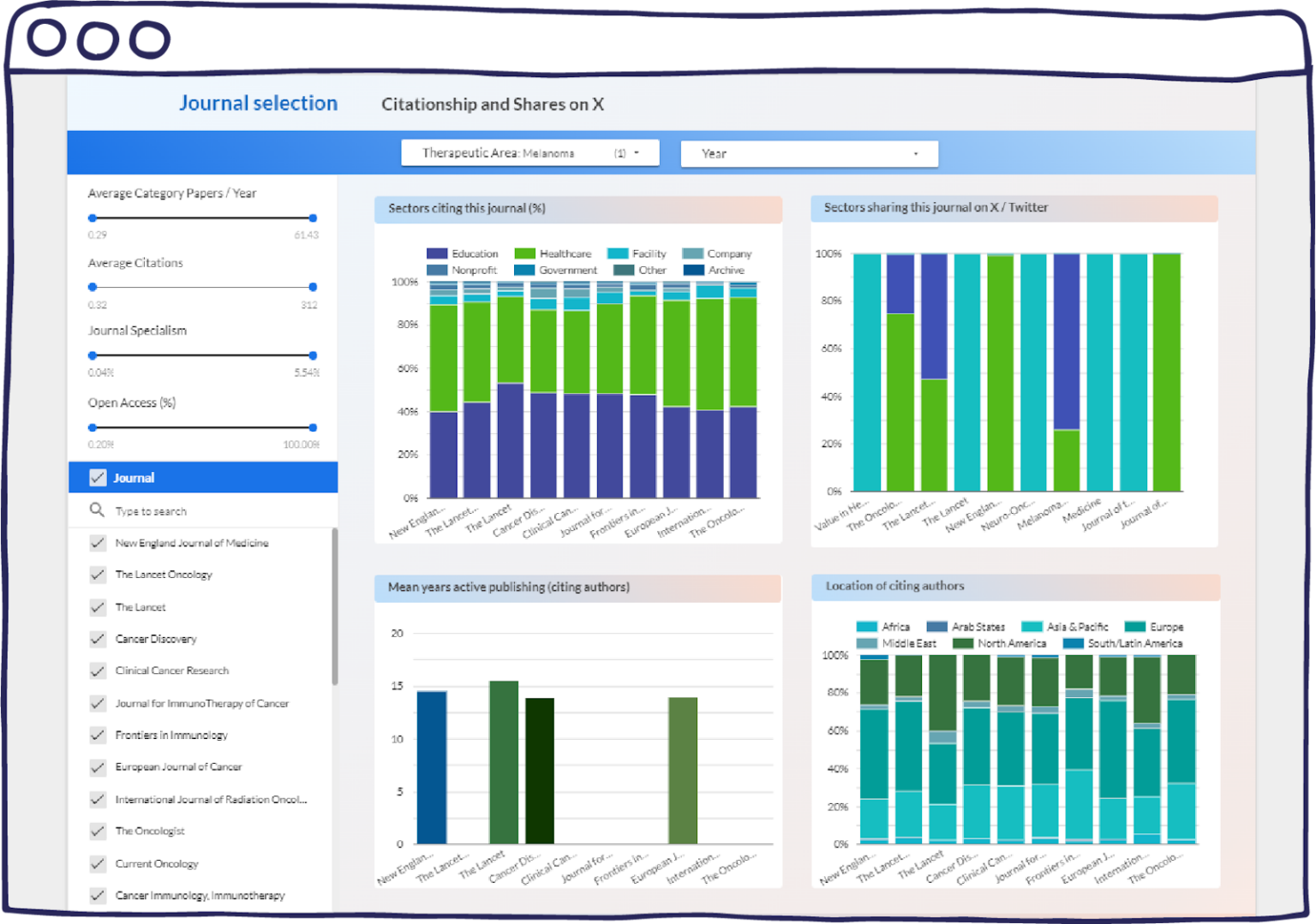
Figure 3: “Citationship and Shares on X”. A screenshot captured from the demo of the Journal Selection dashboard.
HOT TIPS
What look out for while selecting a good journal
-
Is the journal Open Access ? (it’s a rough rule of thumb that OA – on average – does twice as well as non-OA, like-for-like)
-
Has the journal got a good track record in publishing papers by corporate affiliated authors, do they accept PLSs or other document extenders?
-
Is the editorial board relevant, and do they publish in their own journal ?
-
Is the webpage clear, easy to use, and can you find it in search engines?
-
Does the journal produce a good impact within the research area you are working on?
Measuring real-world impact
The journey of a research output does not end with publication. It is essential to measure the effectiveness of research in aiding healthcare professionals’ informed clinical decisions. That is where solutions such as the Publication Impact dashboard easily measure a publication’s impact across various metrics.
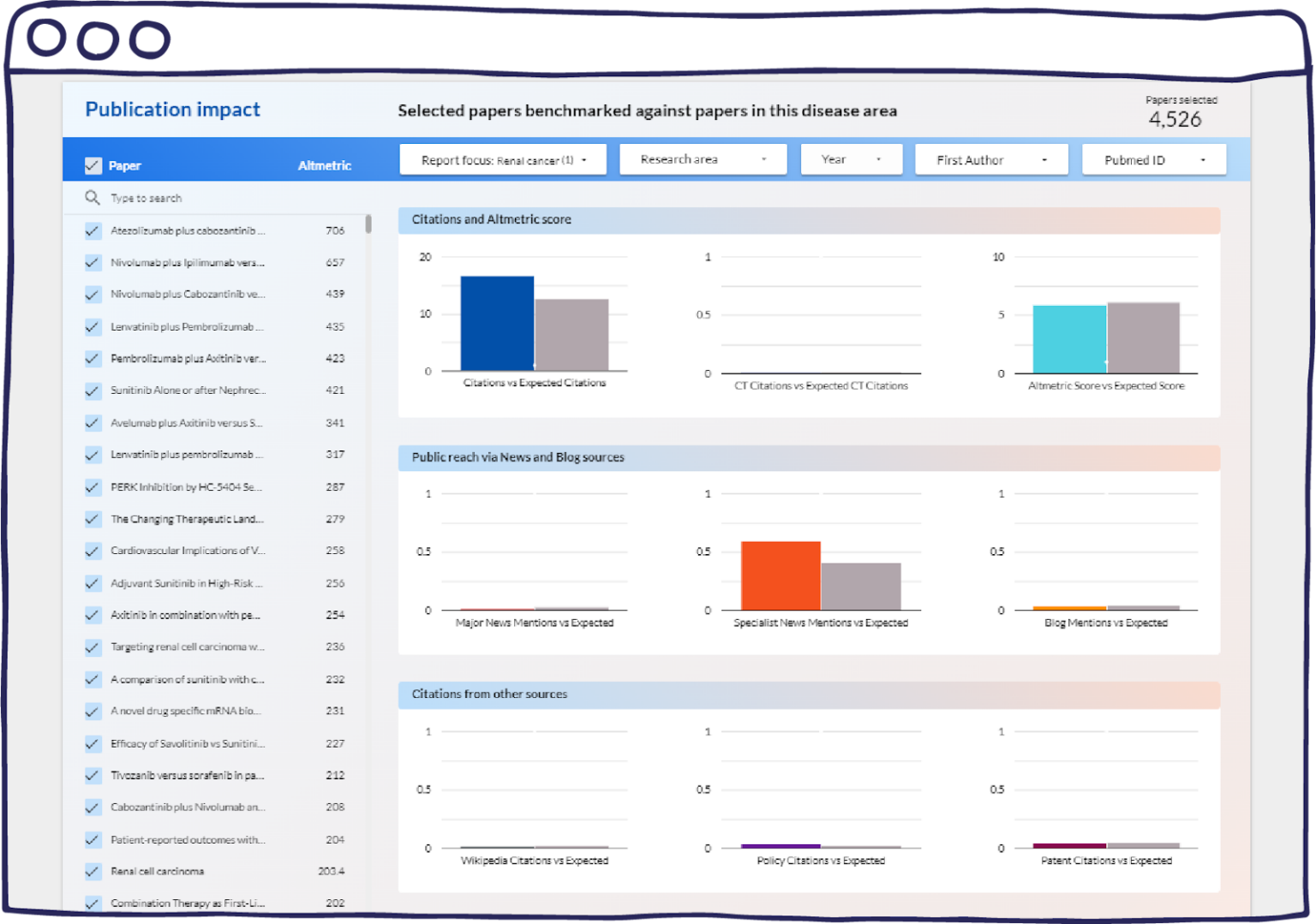
Figure 4: “Selected papers benchmarked against papers in this disease are”. A screenshot captured from the demo of the Publication Impact dashboard.
By combining data from Dimensions and Altmetric, this tool provides stakeholders with an accurate assessment of a publication’s performance, allowing comparisons with similar research outputs and reaching target audiences.The dashboard provides direct insights into how pharmaceutical publications are effectively aiding healthcare professionals in making informed clinical decisions. This enables Medical Affairs teams to monitor the effectiveness of their communications and publications strategies and gauge whether a publication is effectively influencing patient care. By understanding the concrete outcomes of research, stakeholders can pinpoint areas needing enhancement and allocate resources strategically to maximize impact.
Adopting a data-driven approach
As we navigate the new complexities of medical research publication, adopting a data-driven approach could hold the key to research outcomes translating into positive patient outcomes. With solutions such as the Journal Selection dashboard and Publication Impact dashboard, stakeholders can track impact and help drive meaningful advancements in healthcare. As the industry continues to evolve, it will become more and more important to harness the power of data to shape the future of scholarly communication in the field of medical research.
For more insights on improving patient outcomes through strategic planning, get in touch with the team at Altmetric.



